On 31 December 2019, the WHO China Country Office was informed of cases of pneumonia unknown etiology(unknown cause) detected in Wuhan City, Hubei Province of China. From 31 December 2019 through 3 January 2020, a total of 44 case-patients with pneumonia of unknown etiology were reported to WHO by the national authorities in China. During this reported period, the causal agent was not identified.
On 11 and 12 January 2020, WHO received further detailed information from the National Health Commission China that the outbreak is associated with exposures in one seafood market in Wuhan City.
The Chinese authorities identified a new type of coronavirus, which was isolated on 7 January 2020.[1] This was the start of the recent ongoing pandemic. To date, over 30.6 million COVID-19 cases and 950 000 deaths have been reported to WHO. From 14 through 20 September, there were almost 2 million new cases of COVID-19, which represents a 6% increase compared to the previous week, and the highest number of reported cases in a single week since the beginning of the epidemic. [2]
Analysis of COVID-19 and SARS-CoV-2 Analysis of COVID-19 and SARS-CoV-2 Analysis of COVID-19 and SARS-CoV-2 Analysis of COVID-19 and SARS-CoV-2 Analysis of COVID-19 and SARS-CoV-2 Analysis of COVID-19 and SARS-CoV-2 Analysis of COVID-19 and SARS-CoV-2 Analysis of COVID-19 and SARS-CoV-2 Analysis of COVID-19 and SARS-CoV-2 Analysis of COVID-19 and SARS-CoV-2 Analysis of COVID-19 and SARS-CoV-2 Analysis of COVID-19 and SARS-CoV-2 Analysis of COVID-19 and SARS-CoV-2
This post is an upload of my Class 12th Biology Investigatory project. It provides a comprehensive analysis of COVID-19 and SARS-CoV-2; ranging from its classification, structure and origin to is symptoms, diagnosis, and high risk populations.
Join Weekly Health Newsletter
Every week, I will share with you information about Natural Health, Diet, Exercise, Yoga & Wellness.
CLASSIFICATION AND NOMENCLATURE
A virus is a an ultramicroscopic, metabolically inert, obligate parasites that replicates only within the cells of living hosts, composed of an RNA or DNA core, a protein coat, and, in more complex types, a surrounding envelope.
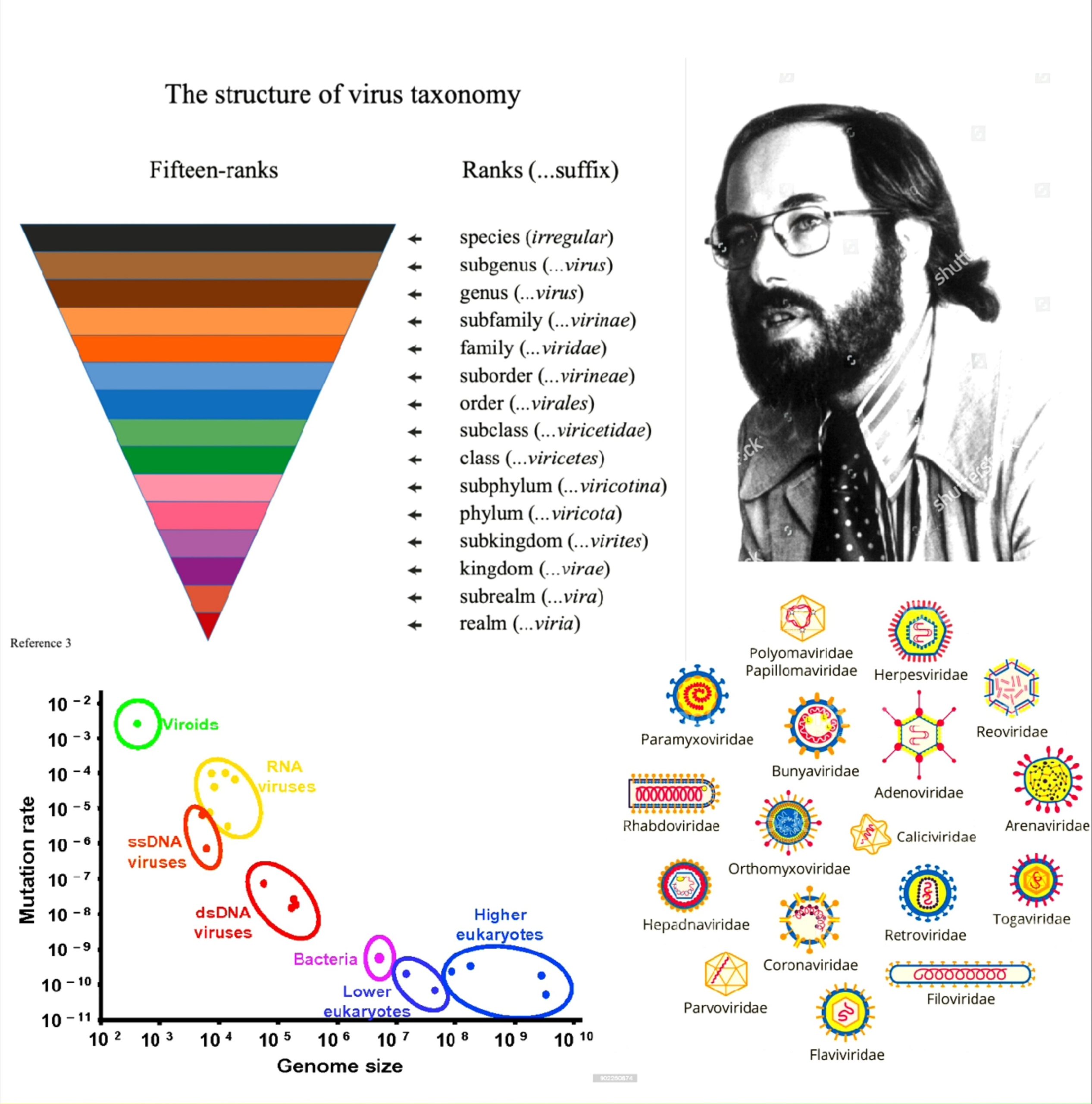
The International Committee on Taxonomy of Viruses (ICTV) is concerned with the designation and naming of virus taxa (i.e. species, genus, family, etc.) rather than the designation of virus common names or disease names. The virus' are classified according to the following features :
- Virion morphology, including size, shape, type of symmetry, presence or absence of peplomers, and presence or absence of membranes.
- Virus genome properties, including type of nucleic acid (DNA or RNA), size of genome, strandedness (single or double), whether linear or circular, sense (positive, negative, ambisense), nucleotide sequence, and presence of special features (repetitive elements, isomerization, 5′-terminal cap).
- Genome organization and replication, including gene order, number and position of open reading frames, a strategy of replication (patterns of transcription, translation), and cellular sites (accumulation of proteins, virion assembly, virion release).
- Virus protein properties, including number, size, and functional activities of structural and nonstructural proteins, amino acid sequence, modifications (glycosylation, phosphorylation, myristylation), and special functional activities (transcriptase, reverse transcriptase, neuraminidase, fusion activities).
- Antigenic properties.
- Physicochemical properties of the virion, including molecular mass, buoyant density, pH stability, thermal stability, and susceptibility to physical and chemical agents, especially ether and detergents.
But, the most commonly used system of virus classification was developed by Nobel Prize-winning biologist David Baltimore in the early 1970s.
BALTIMORE CLASSIFICATION :
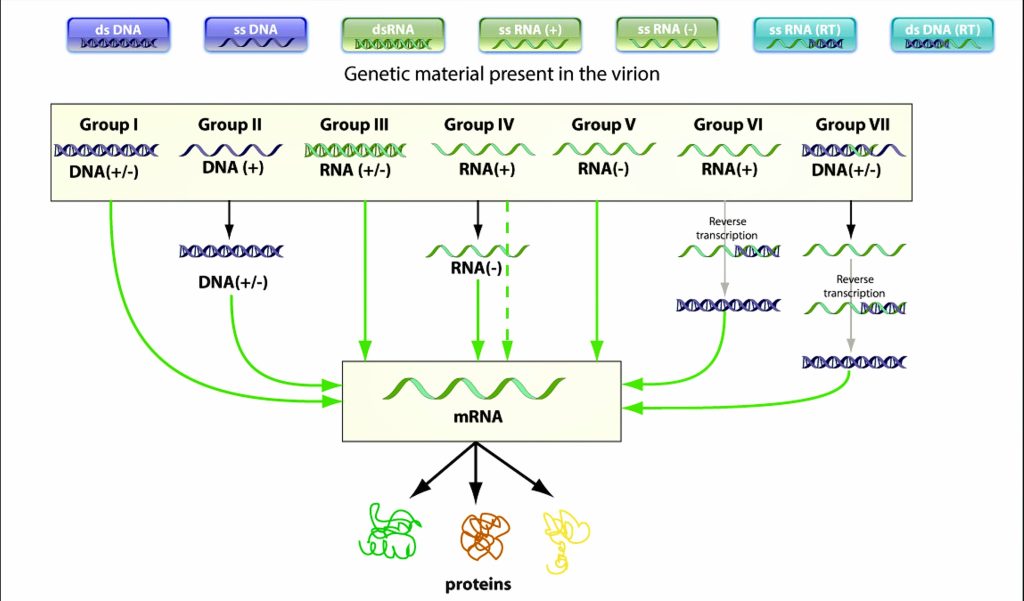
Fig 4.1-1 Baltimore Classsification
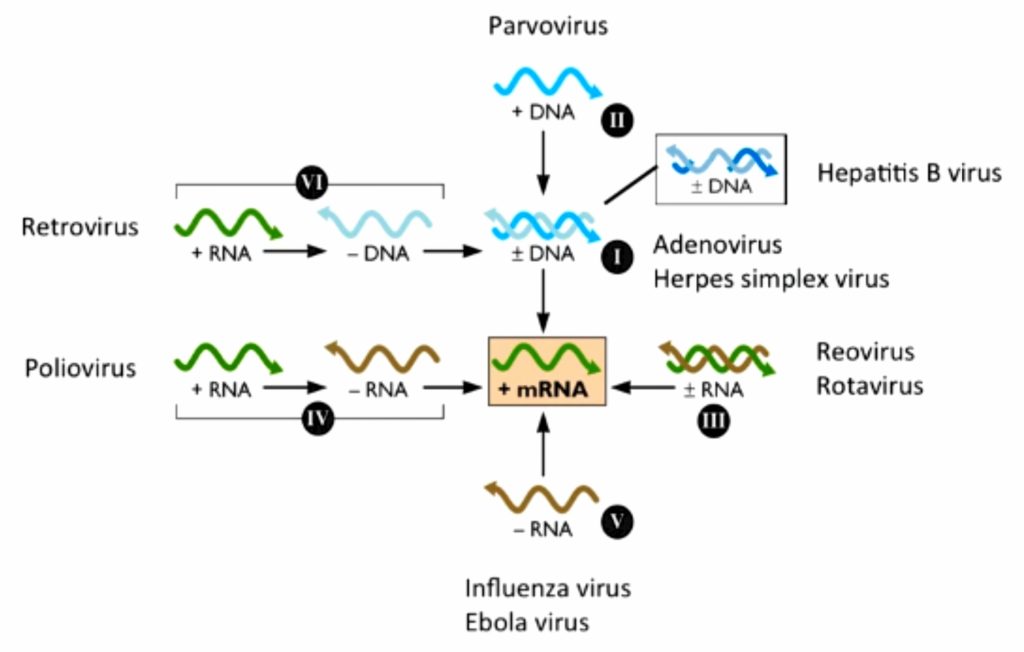
Fig 4.1-2 Baltimore Classsification as mRNA Synthesis
Virologist & Nobel laureate David Baltimore developed a classification which mainly focusses on the central line below - [3] (Fig : 4.1-1, 4.1-2)
'All viruses must synthesize positive-strand mRNAs from their genomes, in order to produce proteins and replicate themselves'
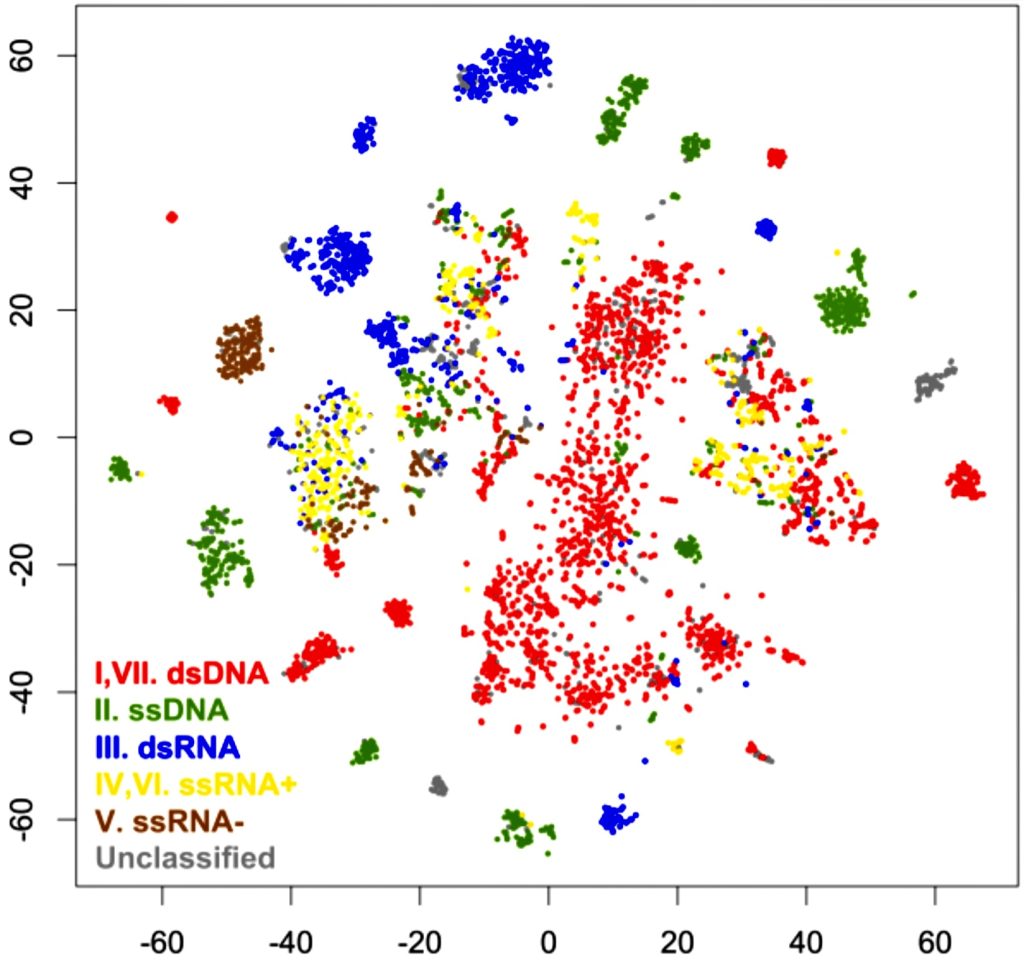
Fig 4.1-3 Plots of VIruses, colored by Baltimore Classification
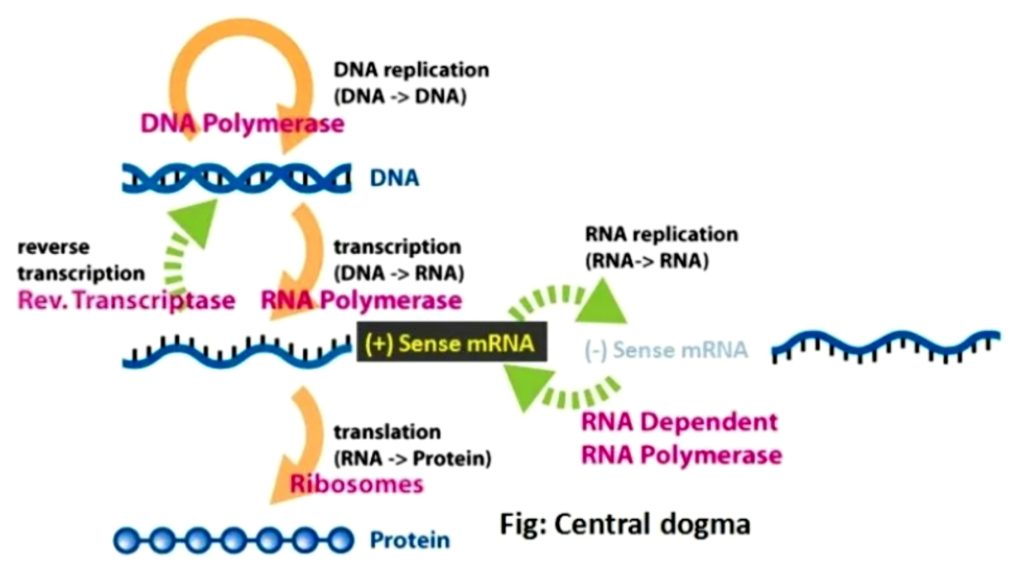
Fig 4.1-4 Central Dogma
The classification divides viruses into seven groups, depending upon type of nucleic acid (ss or ds, RNA or DNA), sense and method of replication. They are: [4][5]
Replication occurs in the nucleus, involving the formation of a (-) sense strand, which serves as a template for (+) strand RNA and DNA synthesis.
E.g., Parvoviruses
Double stranded RNA viruses replicate in the core capsid in the host cell cytoplasm and do depend as heavily on host polymerases as DNA viruses.
These viruses have segmented genomes. Each genome segment is transcribed separately to produce monocistronic mRNAs.
Eg., Reoviruses
Class IV ssRNA viruses have positive-sense RNA genomes, meaning they can be directly read by ribosomes to translate into proteins. They are further divided into viruses with polycistronic mRNA and those with complex transcription.
Examples of some Class IV viruses are Coronaviridae, Flaviviridae, Astroviridae, and Picornaviridae.
Class V viruses have a negative-sense RNA genome, meaning they must be transcribed by a viral polymerase to produce a readable strand of mRNA. The genomes of Class V viruses may be segmented or non-segmented.
Some viruses in Class V are Orthomyxoviridae, Paramyxoviridae, and Rhabodviridae.
Group VI viruses have a positive sense, single-stranded RNA genome, but replicate through a DNA intermediate. The RNA is converted to DNA by reverse transcriptase and then the DNA is spliced into the host genome for subsequent transcription and translation using the enzyme integrase.
Group VI includes retroviruses such as HIV, as well as Metaviridae and Pseudoviridae.
Class VII viruses have a double-stranded DNA genome, but unlike Class I viruses, they replicate via a ssRNA intermediate.
The dsDNA genome is gapped, and subsequently filled in to form a closed circle serving as a template for production of viral mRNA.
To reproduce the genome, RNA is reverse transcribed back to DNA. Hepatitis B virus is a Class VII virus
CLASSIFICATION AND NOMENCLATURE OF CORONAVIRUS :

Fig 4.2-5 Clasification of SARS-CoV-2
During the initial outbreak in Wuhan, China, various names were used for the virus; some names used by different sources included the "coronavirus" or "Wuhan coronavirus".
In January 2020, the World Health Organisation recommended "2019 novel coronavirus" (2019-nCov) as the provisional name for the virus. This was in accordance with WHO's 2015 guideline against using geographical locations, animal species, or groups of people in disease and virus names.
On 11 February 2020, the International Committee on Taxonomy of Viruses adopted the official name "severe acute respiratory syndrome coronavirus 2" (SARS-CoV-2). To avoid confusion with the disease SARS, the WHO sometimes refers to SARS-CoV-2 as "the COVID-19 virus" in public health communications and the name HCoV-19 was included in some research articles.
So, of the realm Riboviria and order Nidovirales, the family Coronaviridae is classified as Group IV – helical enveloped ssRNA type virus. This family is further divided into two sub-families Letovirinae and Orthocoronavirinae. Orthocoronavirinae is classified into four genera – Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus (based on host).
The corona virus, of genus Betacoronavirus, is named as "severe acute respiratory syndrome coronavirus 2" (SARS-CoV-2). And the species to which the virus SARS-CoV-2 belongs is Severe acute respiratory syndrome-related coronavirus. The disease name has been designated as COVID-19 by the WHO. The '19' in COVID-19 stands for the year, 2019, that the virus was first seen. The number '19' has nothing whatsoever to do with virus strains, genotypes, or anything else related to the virus' genetics.[6]
STRUCTURAL BIOLOGY

Since January, structural biologists have been busy modeling the virus' vital proteins, which could lead to therapeutic breakthroughs. As soon as the genomic sequence of SARS-CoV-2, the virus that causes the COVID-19 disease, was mapped, researchers were off to the races in synthesizing its proteins and determining their structures.
Unlike the highly complex genome sequence encoded in human DNA, the new coronavirus has a much shorter sequence and stores its genetic information in a single strand of RNA.

Fig 5-6 Detailed of SARS-CoV-2
The first structural models were uploaded to the Protein Data Bank, an international database for 3D structural data of large biomolecules, within five weeks of the earliest reported cases of COVID-19.
Severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) are both similar to the novel coronavirus, which scientists used as a basis for identifying protein sequences and shapes.
X-ray crystallography had been used rapid and accurate data gathering., and was a key tool in structural biology. However, X-ray crystallography doesn't work for every protein, and thus a new technology was used for the study. The relatively new cryo-electron microscopy emerged as a standout method for capturing the proteins in SARS-CoV-2, reconstructing an array of 2D images into a clear 3D model which facilitated the study.
SARS-CoV-2 is an enveloped, non-segmented, positive sense RNA virus with a diameter of about 65–125 nm, containing single strands of RNA and provided with crown-like spikes on the outer surface. The proteins of SARS-CoV-2 consist of two large polyproteins: ORF1a and ORF1ab (that proteolytically cleave to form 16 nonstructural proteins), four structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N), and six accessory proteins: ORF3a, ORF6, ORF7a, ORF7b, ORF8, and ORF10. The non structural proteins are NSP1. NSP2, NSP3, NSP4, NSP5, NSP6, NSP7, NSP8, NSP9, NSP10, NSP11, NSP12, NSP13, NSP14, NSP15, and NSP16.
STRUCTURAL PROTEINS :

Fig 5.1.1-7 : Lateral view (a), top view (b) and bottom view (c) of the SARS-CoV-2 spike protein trimer Fig 5.1.2-8 : N protein of SARS-CoV-2
![Fig 5.1.3-9 : Tetrameric assembly of the E protein of SARS-CoV-2 Fig 5.1.4-10 : M protein [blue columns] of SARS-CoV-2 attached to other structural proteins Fig 5.1.3-9 : Tetrameric assembly of the E protein of SARS-CoV-2 Fig 5.1.4-10 : M protein [blue columns] of SARS-CoV-2 attached to other structural proteins](https://devadityatomar.com/wp-content/uploads/Screenshot_20221003_230025_Drive-1-1024x509.jpg)
Fig 5.1.3-9 : Tetrameric assembly of the E protein of SARS-CoV-2 Fig 5.1.4-10 : M protein [blue columns] of SARS-CoV-2 attached to other structural proteins
Spike or S Glycoprotein
The spike or S glycoprotein is a transmembrane protein, with a molecular weight of about 150 kDa, is found in the outer portion of the virus. S protein forms homotrimers protruding on the viral surface and facilitates binding of envelope viruses to host cells via angiotensin-converting enzyme 2 (ACE2). This glycoprotein is cleaved by the host cell furin-like protease into 2 sub units namely S1 and S2. [7]
Nucleocapsid or N Protein
The nucleocapsid, known as N protein, is the structural component of CoV localizing in the endoplasmic reticulum-Golgi region that structurally is bound to the nucleic acid material of the virus. Because the protein is bound to RNA, the protein is involved in processes related to the viral genome, the viral replication cycle, and the cellular response of host cells to viral infections [8]. N protein is also heavily phosphorylated and suggested to lead to structural changes enhancing the affinity for viral RNA [9]
Envelope or E Protein
E protein which is the smallest protein in the SARS-CoV structure that plays a role in several aspects of the virus’ life cycle, such as assembly, budding, envelope formation, and pathogenesis.[8]
Membrane or M Glycoprotein
The membrane or M protein, which is the most structurally structured protein and plays a role in determining the shape of the virus envelope. This protein can bind to all other structural proteins. Binding with M protein helps to stabilize nucleocapsids or N proteins and promotes completion of viral assembly by stabilizing N protein-RNA complex, inside the internal virion.
NON STRUCTURAL PROTEINS OR NSPs :

Fig 5.2-11 : NSP 1-4
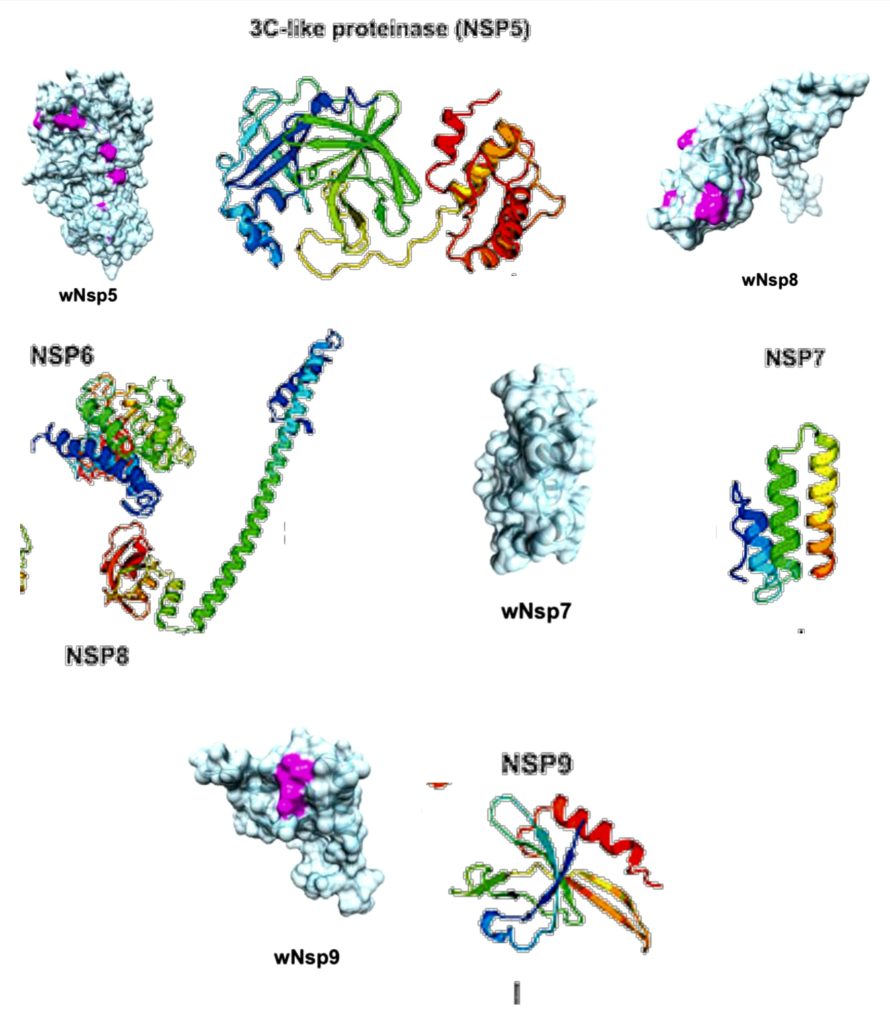
Fig 5.2-12 : NSP 5-9
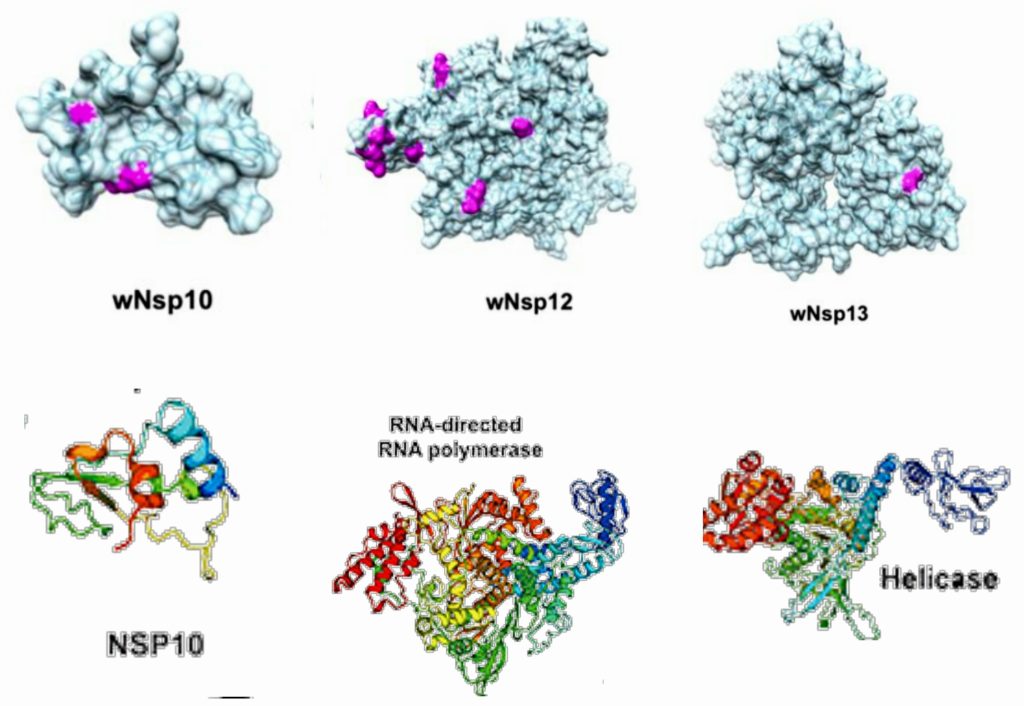
Fig 5.2-13 : NSP 10-13

Fig 5.2-14 : NSP 14-16
This protein is also known as the leader protein. This protein is also found in SARS coronavirus and is known to be a potent inhibitor of host gene expression.
NSP1 binds to the 40S ribosome of the host cell to inactivate translation and promotes host mRNA degradation selectively, while the viral SARS CoV mRNA remain intact.[10]
This protein is conserved in SARS CoV, the related beta coronavirus to SARS CoV-2. In SARS CoV, NSP2 was found to bind to two host proteins: prohibitin 1 and prohibitin 2 (PHB1 and PHB2) [11]. PHB1 and PHB2 proteins are known to play roles in cell cycle progression, cell migration, cellular differentiation, apoptosis, and mitochondrial biogenesis.
The binding of NSP2 to PHB1 and PHB2 proteins suggest that NSP2 plays a role in disrupting the host cell environment.
NSP3 is the papain-like proteinase protein. This protein is nearly 200 kDa in size and is the largest protein (not including the polyproteins ORF1a and ORF1ab) encoded by the coronaviruses.
NSP3 is responsible for the release of NSP1 and NSP2 from the polyproteins 1a and 1ab. [12]
Considering this important protease activity to release essential proteins for viral activity, the inhibition of NSP3 protease activity is an important target for antiviral activity[13]. Tanshinones, a class of natural products have been found to inhibit NSP3 protease activity.
NSP4 interacts with NSP3 and possibly host proteins to confer a role related to membrane rearrangement. Moreover, the interaction between NSP4 and NSP3 is essential for viral replication. [14]
The NSP5 protein based on the Middle East Respiratory Syndrome (MERS) coronavirus has been characterized. NSP5 cleaves at 11 distinct sites to yield mature and intermediate nonstructural proteins (NSPs). [15]
The NSP6 protein of the avian coronavirus (infectious bronchitis virus, IBV) was shown to generate autophagosomes from the endoplasmic reticulum (ER). Autophagosomes facilitate assembly of replicase proteins.
Furthermore, NSP6 limited autophagosome/lysosome expansion, which in turn prevents autophagosomes from delivering viral components for degradation in lysosomes. [16]
NSP7 is required to form a complex with NSP8 (next section) and NSP12 to yield the RNA polymerase activity of NSP8. [17] It has almost 100% similarity with NSP7 of SARS-CoV with only one amino acid residue is different (arginine vs. lysine).
NSP8 is a peptide cofactor that makes a heterodimer with NSP7 (the other peptide cofactor), and this NSP7-NSP8 heterodimer complexes with NSP12.
In addition to the NSP7-NSP8 heterodimer, an NSP8 monomer unit also complexes with NSP12, which ultimately forms the RNA polymerase complex.
The interaction between NSP9 and a cellular protein has been shown to be important for viral replication.
NSP10 has been shown to interact with NSP14 in SARS coronavirus, and this interaction stimulates activity of NSP14. NSP14 is known to function as an S-adenosylmethionine (SAM)-dependent (guanine-N7) methyl transferase (N7-MTase). [18]
Furthermore, NSP10 has also been shown to stimulate the activity of NSP16, which is a 2′-O-methyltransferase. [19]
The function of NSP11 seems to be unknown. NSP11 is made of thirteen amino acids and the first nine amino acids are identical to the first nine in NSP12.
NSP12 is the RNA-dependent RNA polymerase that copies viral RNA. As mentioned, NSP12 makes a complex with an NSP7-NSP8 heterodimer and an NSP8 monomer to confer processivity of NSP12.
NSP12 exhibits poor processivity in RNA synthesis—that is the presence of NSP7 and NSP8 lowers the dissociation rate of NSP12 from RNA. [20]
NSP13 is the helicase enzyme that unwinds the duplex RNA. It has been shown that binding of NSP12 with NSP13 can enhance the helicase activity of NSP13.
In addition to its helicase activity, NSP13 of SARS CoV is also known to possess 5′-triphosphatase activity, which is responsible for introducing the 5′-terminal cap of the viral mRNA.[21] This 5′-terminal cap is the site of recognition for translation and plays a role in splicing, nuclear export, translation, and stability of mRNA.
NSP14 from coronavirus is known to have 3′-5′ exoribonuclease activity and N7-methyltransferase activity. [22]
The guanine-N7-methyltransferase activity is part of the process for introducing the 5′-cap of the virus.
NSP15 of SARS coronavirus has been biochemically characterized as an endoribonuclease. The NSP15 protein specifically targets and degrades the viral polyuridine sequences to prevent the host immune sensing system from detecting the virus. [23]
NSP15 uses manganese as a cofactor to promote endoribonuclease activity. [24]
The viral RNA has a 5′-cap, which protects it from mRNA degradation by 5′-exoribonucleases, promotes mRNA translation, and prevents the viral RNA from being recognized by innate immunity mechanisms. [25]
The RNA cap is an N7-methylated guanine nucleotide connected through a 5′-5′ triphosphate bridge to the first transcribed nucleotide (adenine). NSP16 methylates the 2′-hydroxy group of adenine using S-adenosylmethionine as the methyl source.
ACCESSORY PROTEINS :
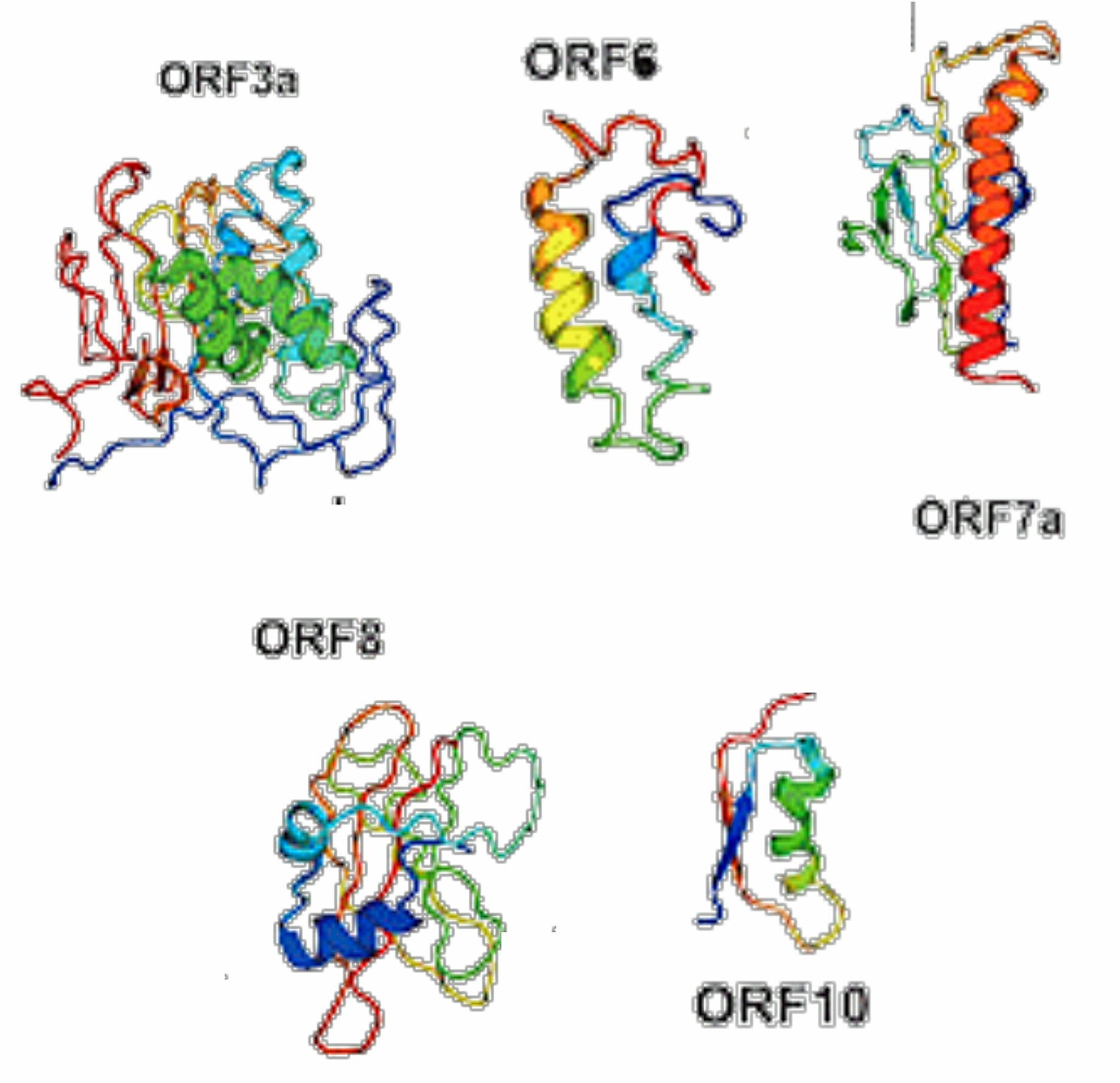
Fig 5.3-15 : Accessory Proteins
The ORF3a protein from SARS CoV is an ion channel protein.
The ORF6 protein from SARS coronavirus is an accessory protein that plays an important role in viral pathogenesis. [26] [27]
Using a yeast two-hybrid system, ORF6 was shown to interact with NSP8, the nonstructural protein related to promoting RNA polymerase activity. [26]
ORF7a from SARS coronavirus is an accessory protein that is a transmembrane protein.
The ORF7b accessory protein from SARS coronavirus is localized in the Golgi compartment. [28]
SARS CoV-2 has a single ORF8 protein which aids in inactivating the interferon signaling.
ORF10 protein from SARS CoV-2 is comprised of 38-amino acids and its function is unknown.
GENETIC MATERIAL

Fig 6-16 : Schematic Presentation of Structure and Genome organisation of SARS-CoV-2
The SARS-CoV-2 virus belongs to the B lineage of the β-coronaviruses (β-CoVs); this family comprises an enveloped, non-segmented, positive-sense single-stranded RNA virus genome, with a 5′ cap structure and 3′ poly-A tail, allowing to perform as an mRNA for translation of the replicase poly proteins.
Among the known RNA viruses, coronaviruses, which are single-stranded and positive-sense RNA viruses have the largest genome between other RNA viruses, with the GC content ranging from 32% to 43%. [29] [30] The genomic organization includes 5′- leader sequence - ORF1/ab - S - ORF3a - E - M - ORF6a - ORF7a - ORF7b - ORF8 - N - ORF10 -3′ from left to right and lacks the hemagglutinin-esterase gene which is detected in some β-CoVs. [31]
About two-thirds of SARS-CoV-2's RNA comprises ORF1a/b region, which with 16 non-structural proteins (nsp1-16) is considered as the largest ORF. The remaining one-third of the genome near the 3′-terminus contains ORFs encode structural and accessory proteins. [32]
ZOONOTIC ORIGIN
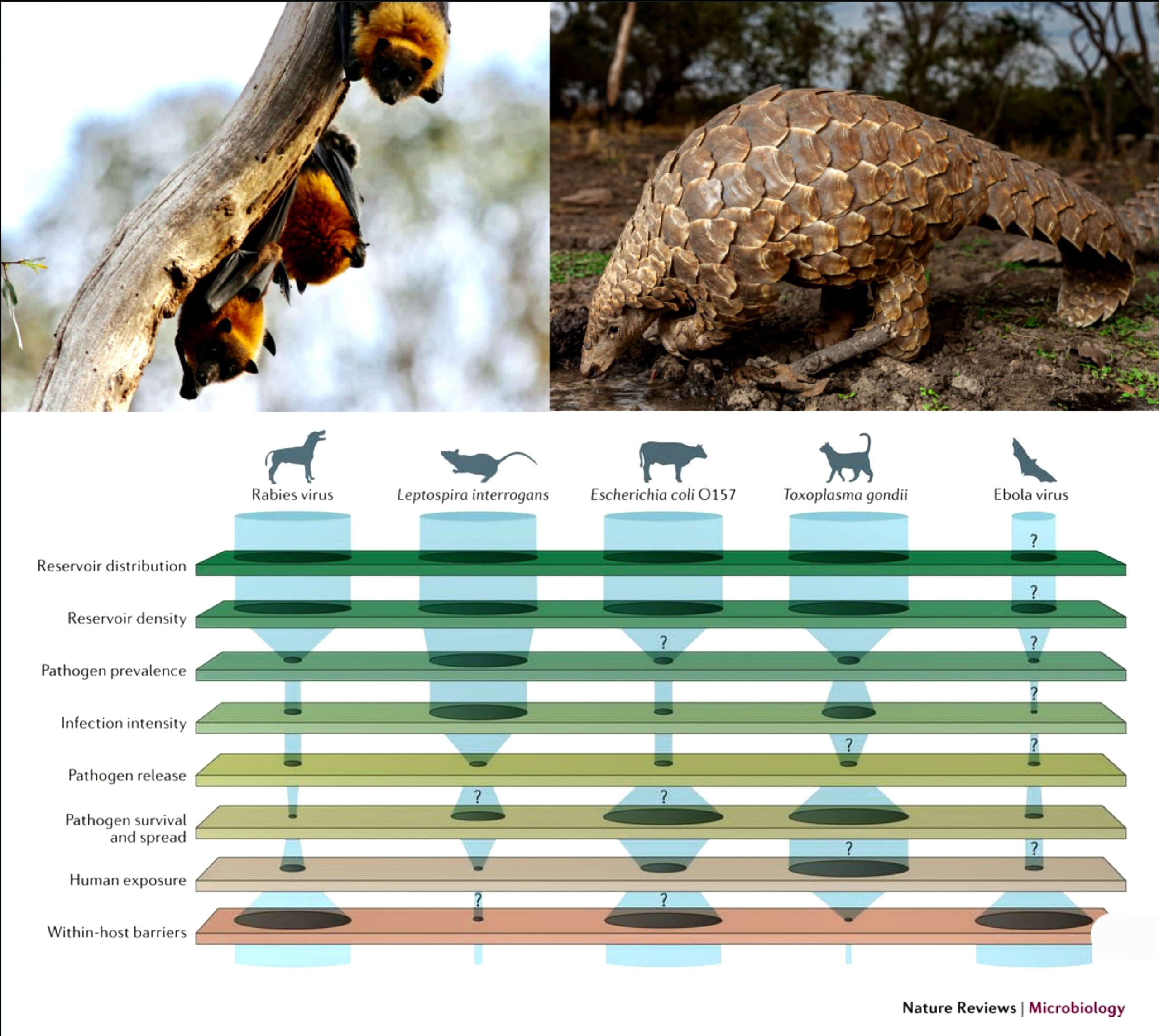
As defined by the WHO, a zoonoses is any disease or infection that is naturally transmissible from vertebrate animals to humans. Some examples are MERS, and SARS-CoV-1.
The word 'zoonoses' is derived from Greek words 'Zoon' meaning animl and 'Noses' meaning disease. The term was first used and coined by Rudolf Virchow, who used it for communicable diseases.
CLASSIFICATION BY RESERVOIR HOST :
Anthropozoonoses
- Infections transmitted to humans from lower vertebrates.
- Eg - Rabies, Plague, Leptospirosis, etc.
Zooanthroponoses
- Infections transmitted from humans to lower vertebrates.
- Eg - Streptococci, Staphylococci, Diphtheria, Human Tuberculosis in parrots and cattles, etc.
Amphixenoses
- Infections maintained in both humans and lower vertebrates and transmitted in either direction.
- Eg - Salmonellosis, etc.
CLASSIFICATION BY MODE OF TRANSMISSION :
Direct Zoonoses
- From an infected vertebrate host to a susceptible host [human] by direct contact, or fomite transmission.
- Eg - Rabies, Anthrax, Leptospirosis, etc.
Cyclozoonoses
- Requires more than one vertebrate host species, but no invertebrate needed for completion of life cycle.
- Eg - Taeniasis
Metazoonoses
- Transmitted biologically by invertebrate vectors, in which the agent multiplies and(or) develops.
- Always an extrinsic incubation period before transmission to another vertebrate host.
- Eg - Plague, Leishmaniasis, etc.
Saprozoonoses
- Requires a vertebrate host and a non-animal developmental site [like soil, plant material, pigeon dropping etc] for the development of the infectious agent.
- Eg - Histoplasmosis, Zygomycosis, etc.
TRANSMISSION OF ZOONOSES :
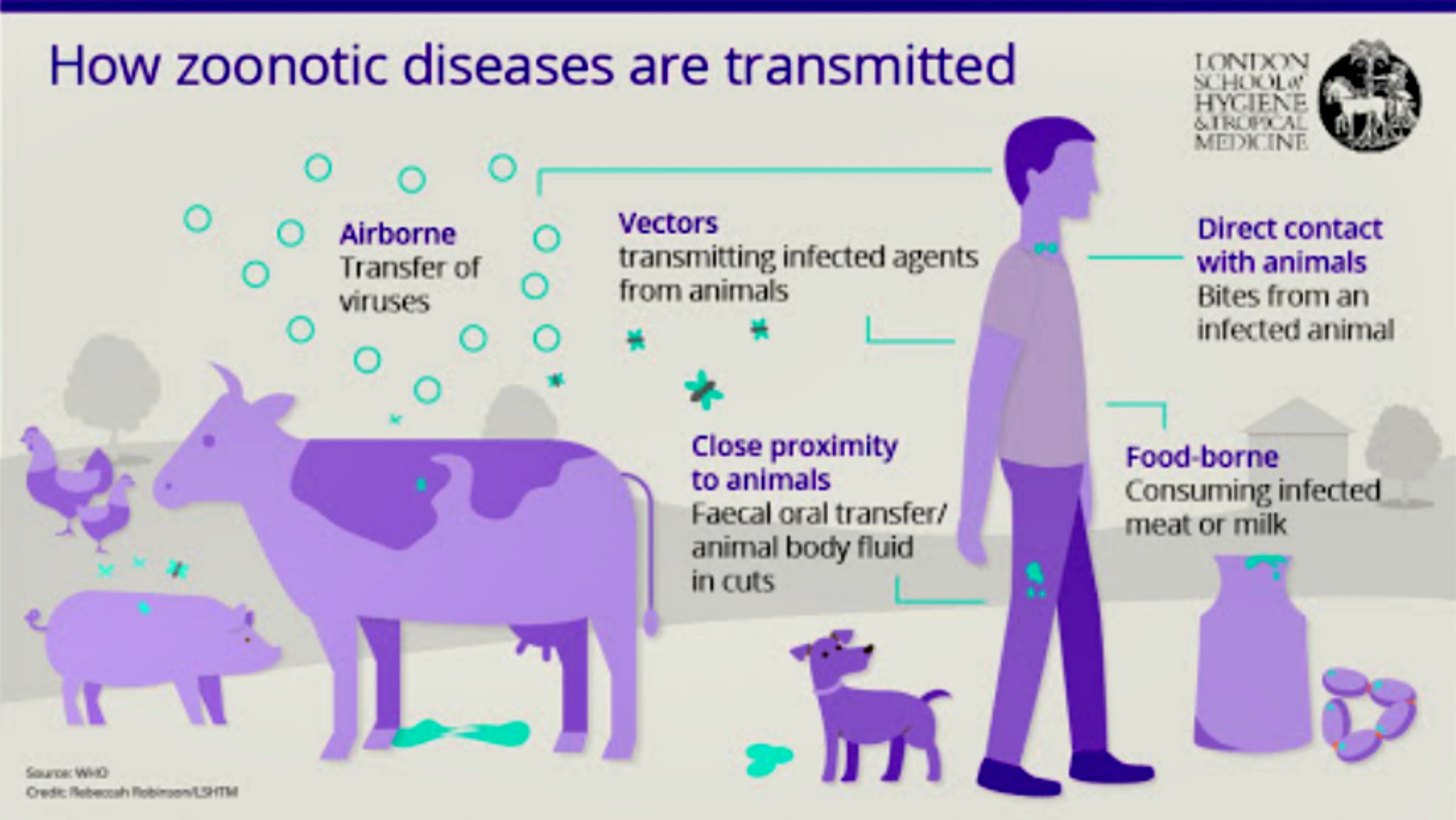
Fig 7.3-17 : Transmission of Zoonoses
People tend to get infected with zoonotic diseases by five major ways - (Fig : 7.3-17)
Direct Contact
- First is by coming in direct contact with saliva, blood, urine, mucous, feces, or other body fluids of an infected animal.
- Examples include petting or touching animals, and bites or scratches.
Indirect/Fomite
- Encountering areas where animals live and roam, or objects or surfaces that have been contaminated with germs.
- Examples include aquarium tank water, pet habitats, chicken coops, barns, plants, and soil, as well as pet food and water dishes.
Vector or Water or Food Borne
- Other way could be the transmission by vectors like a tick, mosquito or a flea.
- The most common way is via faeco-oral route, i.e. the disease could be waterborne or foodborne. [33]
- Eg -
- Being bitten by a tick, or an insect like a mosquito or a flea.
- Eating or drinking something unsafe, such as unpasteurized (raw) milk, undercooked meat or eggs, or raw fruits and vegetables that are contaminated with feces from an infected animal.
- Contaminated food can cause illness in people and animals, including pets.
- Drinking or coming in contact with water that has been contaminated with feces from an infected animal.
ZOONOTIC ORIGIN OF SARS-CoV-2 :
Jumping the Species Barrier
The first patients of SARS-CoV-2 worked in the wet animal markets in Wuhan, China. It is believed that SARS-CoV-2 was introduced from the animal kingdom to human populations during November or December 2019. The spillover of SARS-CoV-2 from animals to humans took place at the beginning of December 2019 and clinical cases appeared at the end of December 2019.
Now, several evidences based on genome sequences, the homology of the ACE2 receptor, and the presence of single intact ORF on gene 8 indicate bats as a natural reservoir of these viruses.
However, an unknown animal is yet to be unravelled as an intermediate host. [36][37]Initial investigations on animal source origin of SARS-CoV-2 have inconclusively revealed snakes, pangolins, and turtles. [34] [35]
Recently, a Pomeranian dog as a probable intermediate host was identified; however, such reports are yet to be validated, and research is underway to explore the emergence of this infectious disease at the animal-human interface. [38][39]
A study reported that the SARS-CoV-2 might infect the cats and further transmitted by the infected cat to other cats. [40] Furthermore, along with dogs and cats, the zoo animals like tigers and lions were also reported to get the SARS-CoV-2 infection and exhibit clinical signs such as vomiting, diarrhoea, dry cough, breathing difficulty and wheezing. [41][42]
Spillover of SARS-CoV-2 was also reported in mink farms of Netherlands, further increasing the concern of transmission to humans.
Zoonotic Spillover
Since the beginning of 2002 till the end of 2019, three coronaviruses viz. SARS-CoV, MERS-CoV, and SARS-CoV-2 have caused havoc in the human population globally and will continue to do so.
Earlier identified betacoronaviruses (SARS-CoV and MERS-CoV) were reported in Guangdong province of China in November 2002 and Saudi Arabia in 2012, respectively SARS-CoV-2 is the third zoonotic betacoronaviruses recognized in this century. Both SARS-CoV and SARS-CoV-2 showed prominent similarities in their pathogenesis and epidemics. In both cases, bats were considered as the natural host, and the cold temperature and low humidity in cold, dry winter provided conducive environmental conditions that promoted the survival of the virus in the environment. [43]
Zoonotic spillover is the transmission of pathogens to humans from vertebrate animals. At present, these spillovers are of significant concern as in the past, many spillovers in the form of Nipah, Hendra, Ebola, SARS, MERS, and ongoing COVID-19 involving many animal species like pigs, horses, monkeys, camels, civets, among others, were documented.
Spillover is governed by the interaction of viral-specific proteins like S protein and host ACE2 receptor. [44] Mutations in amino acid sequences of RBDs [Receptor Binding Domains] results in a change in specificity of a receptor, interaction and binding, hence alteration in transmissibility, pathogenicity and cross-species jumping with a predisposition to novel and more severe diseases. [45]
In the case of SARS-CoV-2, RBD of S protein has 10–15 times affinity ACE2 receptor. [45] The enhancement of pathogenicity has been recorded previously too, which was bestowed upon by intermediate hosts, and thus helped in a spillover event.
Transmission to Humans

Fig 7.4-18 : Transmission to Humans
(Fig : 7.4.3-18) The involvement of intermediate hosts in maintaining and transmitting the virus to susceptible host predisposes humans to novel CoVs leading to the emergence of new diseases in humans.Usually, one or more types of animal hosts are involved in the transmission cycle of CoVs to humans. [44]
Bats have been the natural hosts for most human CoVs of Alphacoronavirus and Betacoronavirus genera. Genome sequence analysis has revealed bats as a natural host for SARS-CoV-2 [44]
In natural or reservoir hosts, CoVs adapts well, however, being unstable RNA viruses, they keep multiplying continuously without producing disease thereby enabling persistence or survivability and accumulation of mutations over the time resulting in the emergence of newer and novel strains of viruses. These unique strains or viruses occasionally spill over to other species including animals or humans, adapting to their body systems and hence broaden the biological host range for evolutionary sustainability; however, results in epidemiological widening of disease sphere as well.
In the context of SARS-CoV-2, snakes, pangolins and bats have been suspected as intermediate hosts since the first cases of COVID-19 had links to Huanan Sea Food Market where different animals, birds, and wild animals were being sold along with seafood items. [46] [47]
Bats are the natural reservoir host of many CoVs.The bat coronavirus, BatCoV RaTG13, has shown higher relatedness to SARS-CoV-2 at the whole genome level and spike gene in particular. [48] Based on Resampling Similarity Codon Usage (RSCU), snakes (Bungarus multicinctus and Naja atra) were suggested as wildlife reservoirs of SARS-CoV-2 and reported to be associated with the cross-species transmission and later it was disapproved by other researchers.
Unfortunately, to date, the intermediate host of the SARS-CoV-2 is abstruse what results in its escalation in the human population around the globe.
INFECTION AND TRANSMISSION
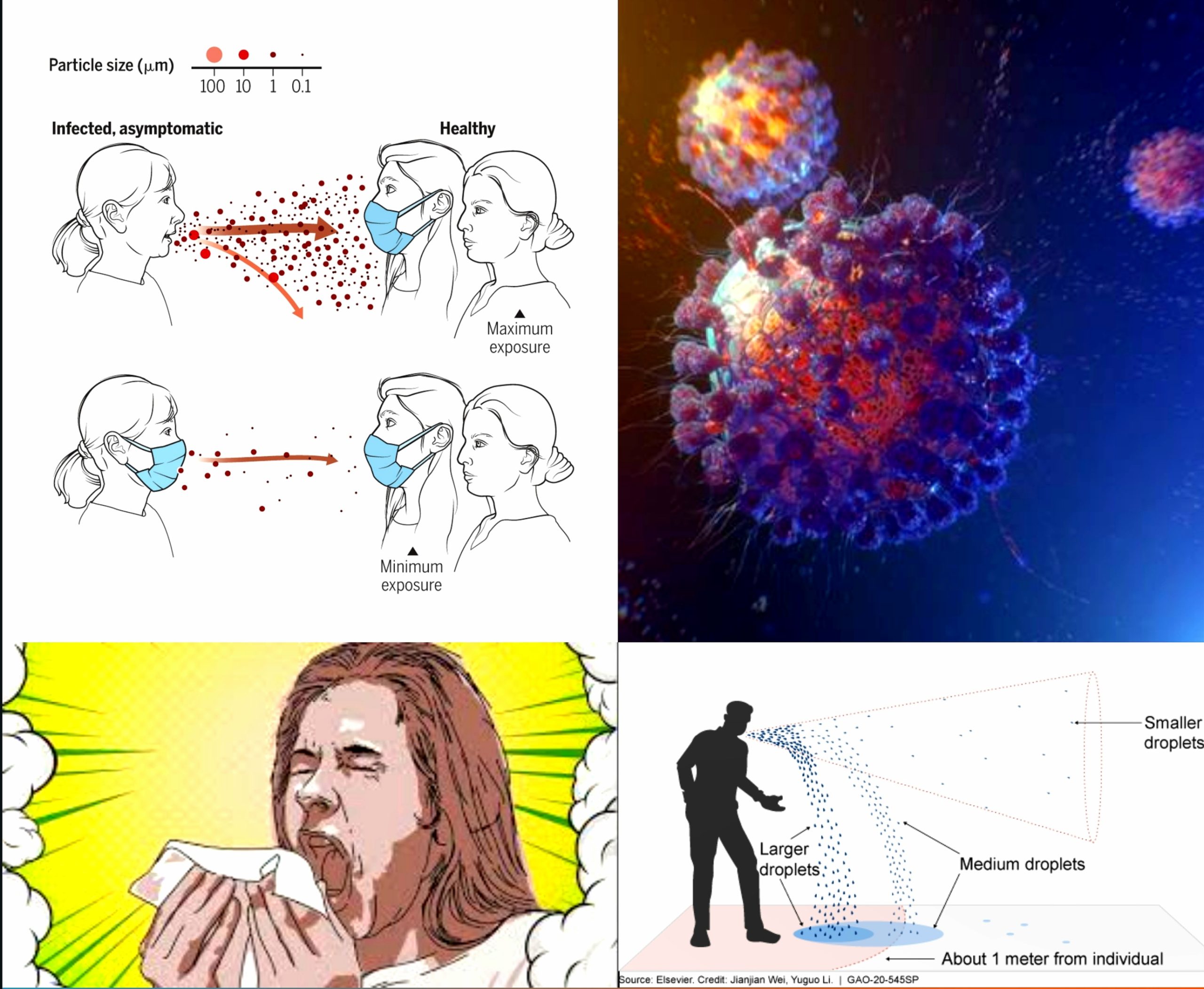
ROUTES OF TRANSMISSION :
Respiratory Droplets and Aerosols
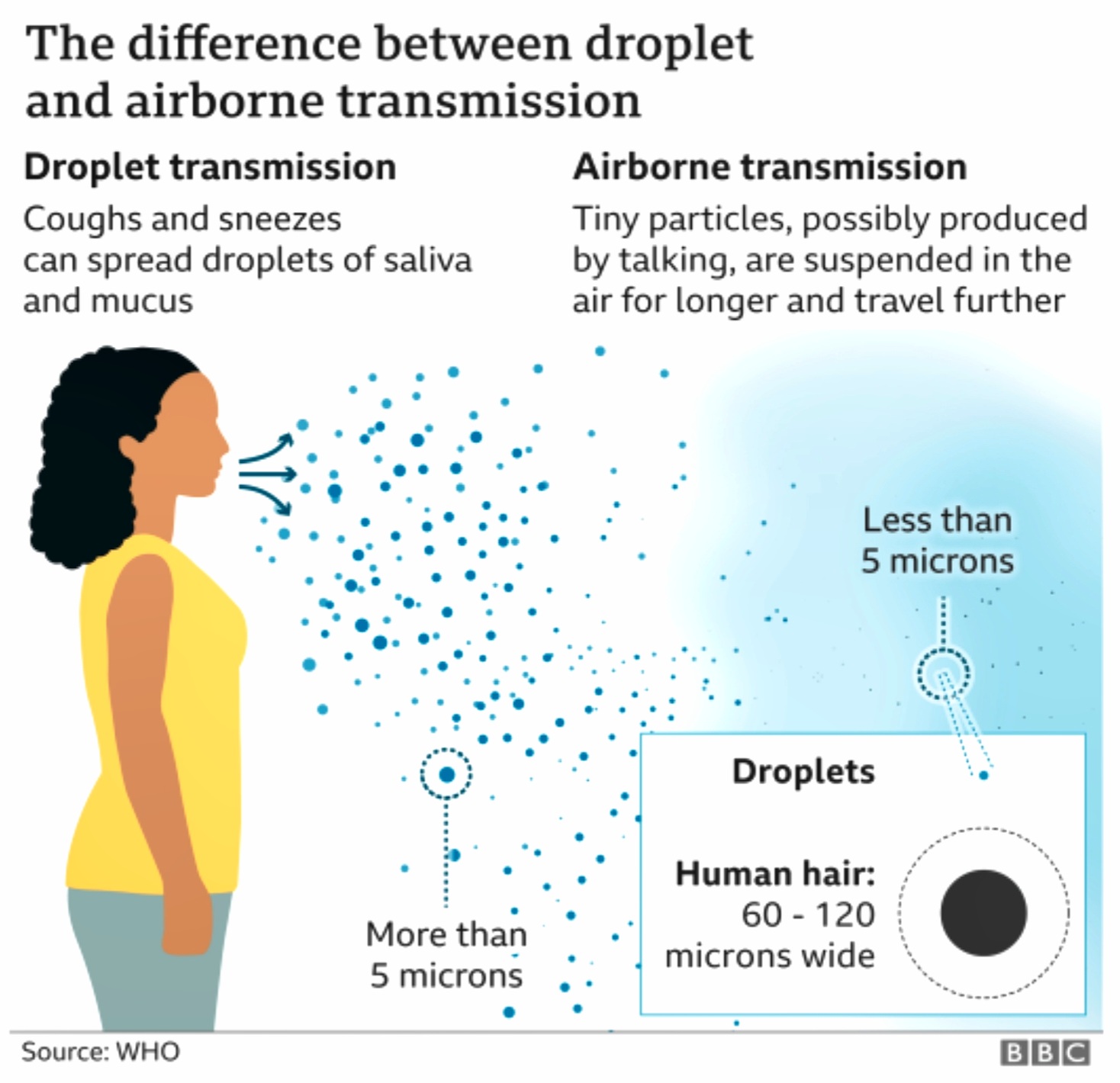
Fig 8.1.1-19 : Respiratory Droplets
(Fig : 8.1.1-19)The SARS-CoV-2 mainly spread from person to another through small respiratory droplets from the nose or mouth when a person confirmed COVID-19 coughs or exhales. These droplets land on objects and surfaces around the person. Other people may catch the virus by breathing in droplets or touching these objects or surfaces, then touching their eyes, nose, or mouth. [49]
These respiratory emissions can range in size, with large droplets typically referring to those above 5-10 µm in diameter, and small droplets or aerosols referring to those below 5-10 µm diameter. Forceful respiratory actions such as coughing and sneezing can produce a burst of droplets that range in size and could include both large droplets and aerosols that present an exposure risk in close proximity to an infected person.
Under experimental conditions, SARS-CoV-2 has been found to remain viable when airborne over short distances for several hours and in field studies, viable virus has been isolated from air samples at distances greater than two metres from a COVID-19 patient. [50]
Fomite Transmission
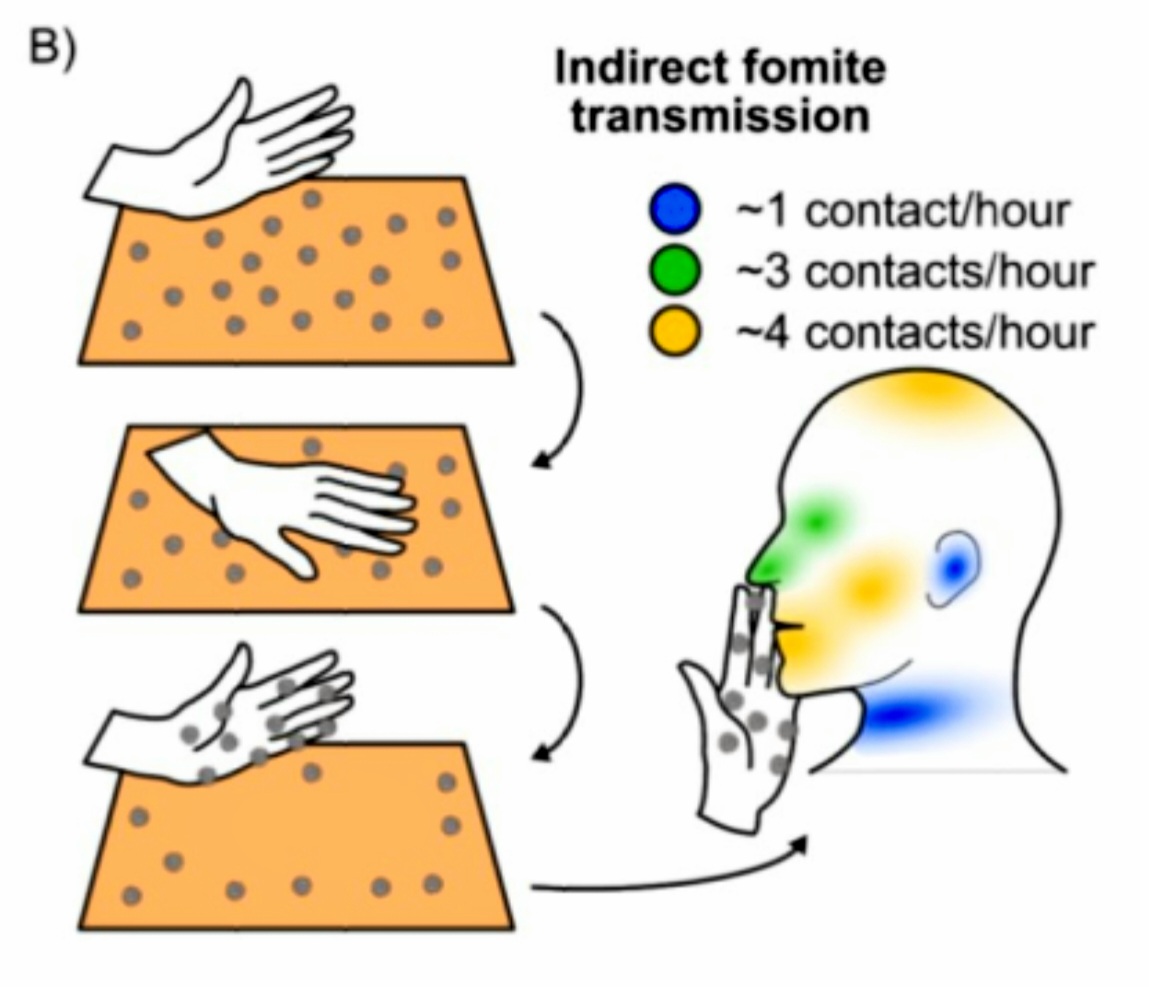
Fig 8.1.2-20 : Indirect Fomite Transmission
(Fig : 8.1.2-20) Contact with contaminated surfaces (fomites) followed by touching of the eyes, mouth or nose is another possible mode of SARS-CoV-2 transmission, although it is not considered to be the main route. Fomites can become contaminated by deposition of droplets, aerosols, sputum or feces, either directly or by cross-contamination by touching an object with contaminated hands. Surfaces that are frequently touched by many people, such as door handles, or faucets may be more important in fomite transmission compared to objects or surfaces that are only touched incidentally and less frequently.
The virus appears to remain viable for longer periods (one to seven days or more) on smooth hard surfaces such as stainless steel, hard plastic, glass, and ceramics and for shorter periods (several hours to two days) on porous materials such as paper, cardboard, and textiles, although viability may be dependent on other factors such as temperature. [51] [52]
Faeco-Oral Route

Fig 8.1.3-21 : Faeco-Oral Transmission
Generally, many respiratory pathogens, such as influenza, SARS-CoV and MERS-CoV, cause enteric symptoms, so is SARS-CoV-2. In early reports from Wuhan, 2–10% of patients with COVID-19 had gastrointestinal symptoms such as diarrhea or vomiting. [53]
The study found that the detection of SARS-CoV-2 nucleic acid positive in a few feces of patients with confirmed COVID-19 cases indicated the presence of a live virus. Considering the evidence of fecal excretion for SARS-CoV-2, the virus can also be transmitted via the fecal-oral transmission route or re-transmitted through the formation of aerosols in virus-containing feces.
Blood-Borne Spread
Cytokines (CK) are key factors regulating the immune response caused by many infectious pathogens that can significantly damage host organs and tissues. In the early stages of SARS-CoV infection, cytokine levels in the blood are rapidly elevated, the elevation of which is associated with the progression of lung invasion and injury [54]
The concentrations of various cytokines in the blood of severe patients with SARS-CoV-2 infection were significantly higher than those of non-serious patients. The concentration of cytokines can indicate the severity of the disease.
In severe cases of infection, immune system was overstimulated, resulting in a cytokine storm that leads to severe immune damage to the lungs and other organs. More and more cases reported that the brain, kidney, and heart impairment induced by the virus infection, may contribute to multi-organ failure and death eventually. [55]
Massive accumulation of SARS-CoV-2 leads to a surge of immune cells and more and more pro-inflammatory cytokines, resulting in a rapid increase in CK levels in the blood, or releasing more virus particles into the blood circulation. The virus and cytokines positively induce high expression of ACE2 in the intestinal epithelium and other organs which accelerated over-expression of ACE2 and viral binding, causing systemic infections with the virus.
PROCESS OF INFECTION :

Fig 8.2.1-22 : Infecting a Human
Introduction
(FIG : 8.2.1-22) Viruses enter cells and initiate infection by binding to their cognate cell surface receptors. The expression and distribution of viral entry receptors therefore regulates their tropism, determining the tissues that are infected and thus disease pathogenesis. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the third human coronavirus known to co-opt the peptidase angiotensin-converting enzyme 2 (ACE2) for cell entry. The interaction between SARS-CoV-2 and ACE2 is critical to determining both tissue tropism and progression from early SARS-CoV-2 infection to severe coronavirus disease 2019 (COVID-19).
Angiotensin-converting enzyme 2 is a zinc membrane-bound metalloproteinase that acts as a carboxypeptidase able to hydrolyze Ang I to Ang-(1-9) and Ang II to Ang-(1-7). ACE converts angiotensin I to angiotensin II, a highly active octapeptide that exhibits both vasopressor (vascular contraction to increase blood pressure) and proinflammatory activities. The carboxypeptidase activity of ACE2 converts angiotensin II to the heptapeptide angiotensin-(1-7), a functional antagonist of angiotensin II.
The Renin Angiotensin System (RAS) seems to be involved in COVID-19 natural course, since studies suggest the membrane-bound Angiotensin-converting enzyme 2 (ACE2) works as SARS-CoV-2 cellular receptor. The renin–angiotensin–aldosterone system (RAAS) is a critical regulator of blood volume and systemic vascular resistance. While the baroreceptor reflex responds in a short-term manner to decreased arterial pressure, the RAAS is responsible for more chronic alterations. It is composed of three major compounds: renin, angiotensin II, and aldosterone. These three act to elevate arterial pressure in response to decreased renal blood pressure, decreased salt delivery to the distal convoluted tubule, and/or beta-agonism. Through these mechanisms, the body can elevate the blood pressure in a prolonged manner.
The ACE2 receptor is present in most organs: it is attached to the cell membrane of mainly lung type II alveolar cells, enterocytes of the small intestine, arterial and venous endothelial cells, and arterial smooth muscle cells in most organs. Since the introduction of virus is mostly through the respiratory pathway, most cases of infection are isolated in those areas.
Entry into the Cell via ACE2
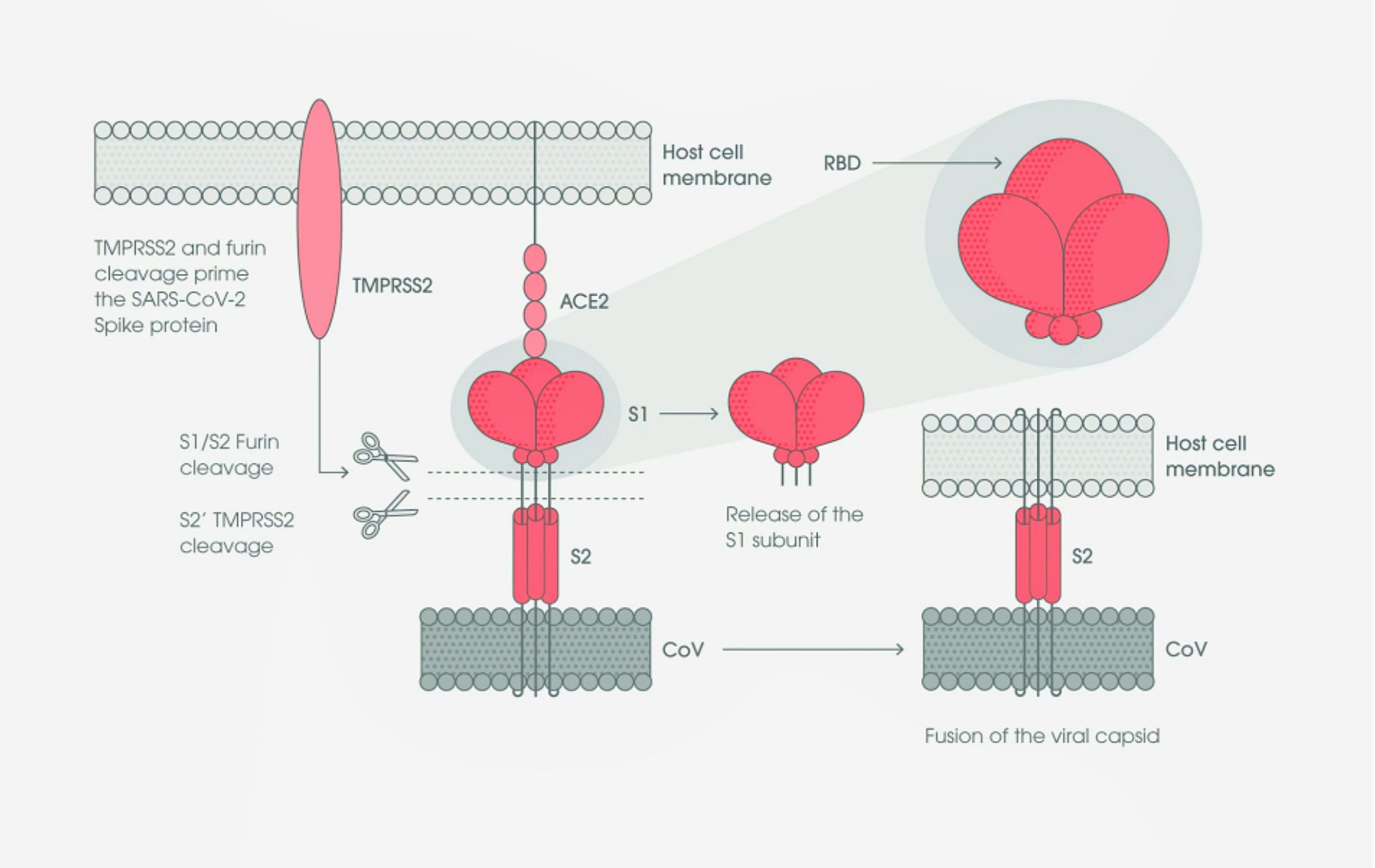
FIG : 8.2.2-23 : Interaction of S protein and ACE-2
(Fig : 8.2.2-23) Like all coronaviruses, SARS-CoV-2 utilizes the S glycoprotein to promote entry into the host cell. This protein contains two functional domains: an S1 receptor-binding domain (RBD), and a second S2 domain that mediates the fusion of the viral and host cell membranes.
SARS-CoV-2 S protein binds to the ACE2 receptor on the host cell, initially through the S1 receptor binding domain. The S1 domain is then shed from the viral surface, allowing the S2 domain to fuse to the host cell membrane. This process is dependent upon activation of the S protein, by cleavage at two sites (S1/S2 and S2’) via the a cellular serine protease, TMPRSS2, and, in a smaller rate, an endosomal cysteine protease, cathepsin B, and L (CatB/L) (Hoffmann et al., 2020)[70].
Furin cleavage at the S1/S2 site may lead to conformational changes in the viral S protein that exposes the RBD and/or the S2 domain. TMPRSS2 cleavage of the SARS-CoV-2 S protein is believed to enable the fusion of the viral capsid with the host cell to permit viral entry[71][72]
Exposure of the RBD in the S1 protein subunit creates an unstable subunit conformation. Consequently, during binding, this subunit undergoes conformational rearrangement between two states, known as the up and down conformations. The down state transiently hides the RBD, while the up state exposes the RBD, but temporarily destabilizes the protein subunit[73][74][75]. Within the trimeric S protein, only one of the three RBD is present in the accessible conformation to bind the human Angiotensin 2 (hACE2) host cell receptor[76].
The critical functions of the SARS-CoV-2 S protein, ACE2, and the Furin and TMPRSS2 enzymes in binding and mediating viral entry to the host cell make these proteins key targets for drug development and viral inhibition in the efforts against COVID-19.
Analysis of COVID-19 and SARS-CoV-2
Viral Gene Expression

FIG : 8.2.3-24 : Transcribed mRNA and its Respective Proteins
(Fig : 8.2.3-24) The release of the coronavirus genome into the host cell cytoplasm upon entry marks the onset of a complex programme of viral gene expression, which is highly regulated in space and time.
The translation of ORF1a and ORF1b from the genomic RNA produces two polyproteins, pp1a and pp1ab, respectively. Sixteen non-structural proteins are co-translationally and post-translationally released from pp1a (nsp1–11) and pp1ab (nsp1–10, nsp12–16) upon proteolytic cleavage by two cysteine proteases that are located within nsp3 (papain-like protease; PLpro) and nsp5 (chymotrypsin-like protease).
Proteolytic release of nsp1 is known to occur rapidly[77], which enables nsp1 to target the host cell translation machinery[78],[79],[80]. Nsp2–16 compose the viral RTC and are targeted to defined sub cellular locations where interactions with host cell factors determine the course of the replication cycle[81],[82],[83]. Nsp2–11 are believed to provide the necessary supporting functions to accommodate the viral RTC (Replication/Transcription Complex), such as modulating intracellular membranes, host immune evasion and providing cofactors for replication, whereas nsp12–16 contain the core enzymatic functions involved in RNA synthesis, RNA proofreading and RNA modification [84],[82].
RNA synthesis is performed by the nsp12 RNA-dependent RNA polymerase (RdRP) and its two cofactors nsp7 and nsp8, the latter with proposed primase or 3′-terminal adenylyltransferase activity[84],[82],[85],[86]. Notably, nsp14 provides a 3′–5′exonuclease activity that assists RNA synthesis with a unique RNA proofreading function[87] .
The establishment of the viral RTC is crucial for virus replication and thus a promising target for antivirals against SARS-CoV-2. One such target is Mpro, which resides in nsp5. Mpro releases the majority of nsps from the polyproteins and is essential for the viral life cycle. Furthermore, as Mpro is very sequence specific, compounds that structurally mimic those cleavage sites can specifically target the viral protease with little or no impact on host cellular proteases[88],[89],[90].
RNA Synthesis

FIG : 8.2.4-25 : Discontinuous RNA Synthesis
(Fig : 8.2.4-25) Viral genomic replication is initiated by the synthesis of full-length negative-sense genomic copies, which function as templates for the generation of new positive-sense genomic RNA. These newly synthesized genomes are used for translation to generate more nsps and RTCs or are packaged into new virions.
A hallmark of coronaviruses and most members of the order Nidovirales is the discontinuous viral transcription process, first proposed by Sawicki and Sawicki[91], that produces a set of nested 3′ and 5′ co-terminal subgenomic RNAs (sgRNAs)[91],[92].
During negative-strand RNA synthesis, the RTC interrupts transcription following the encounter of transcription regulatory sequences (TRSs) that are located upstream to most ORFs in the 3′ one-third of the viral genome. At these TRS elements, also called TRS ‘body’, the synthesis of the negative-strand RNA stops and is re-initiated at the TRS adjacent to a leader sequence (TRS-L) located at about 70 nucleotides from the 5′ end of the genome [91],[92],[93],[94],[95].
This discontinuous step of coronavirus RNA synthesis involves the interaction between complementary TRSs of the nascent negative strand RNA (negative-sense TRS body) and the positive strand genomic RNA (positive-sense TRS-L). Upon re-initiation of RNA synthesis at the TRS-L region, a negative strand copy of the leader sequence is added to the nascent RNA to complete the synthesis of negative-strand sgRNAs.
The discontinuous step of negative strand RNA synthesis results in the production of a set of negative-strand sgRNAs that are then used as templates to synthesize a characteristic nested set of positive-sense sg mRNAs that are translated into structural and accessory proteins. Although the coronavirus sg mRNAs are structurally polycistronic, it is assumed that they are functionally monocistronic.
Expression of Structural and Accessory Proteins
The ORFs encoding the structural proteins (that is, S protein, envelope (E) protein, membrane (M) protein and nucleocapsid (N) protein) are located in the 3′ one-third of coronavirus genomes. Interspersed between these ORFs are the ORFs encoding for so-called accessory proteins.
In general, coronavirus structural proteins assemble and assist in the budding of new virions at the endoplasmic reticulum (ER)-to-Golgi compartment that are suggested to exit the infected cell by exocytosis [96],[97],[98]. These proteins are translated from positive sense sg mRNA.
At least five ORFs encoding accessory genes have been reported for SARS-CoV-2: ORF3a, ORF6, ORF7a, ORF7b and ORF8 (GenBank entry NC_045512.2). In addition, ORF10 has been postulated to be located downstream of the N gene. However, not all of these ORFs have been experimentally verified yet and the exact number of accessory genes of SARS-CoV-2 is still debated [93],[99].
Replication Compartment
After translation, the structural proteins, along with accessory proteins and its genome, form new viruses (virion) capable of infecting other cells. The mechanism of the formation of virions is still being researched and is out of scope of this project.
SYMPTOMS of COVID-19
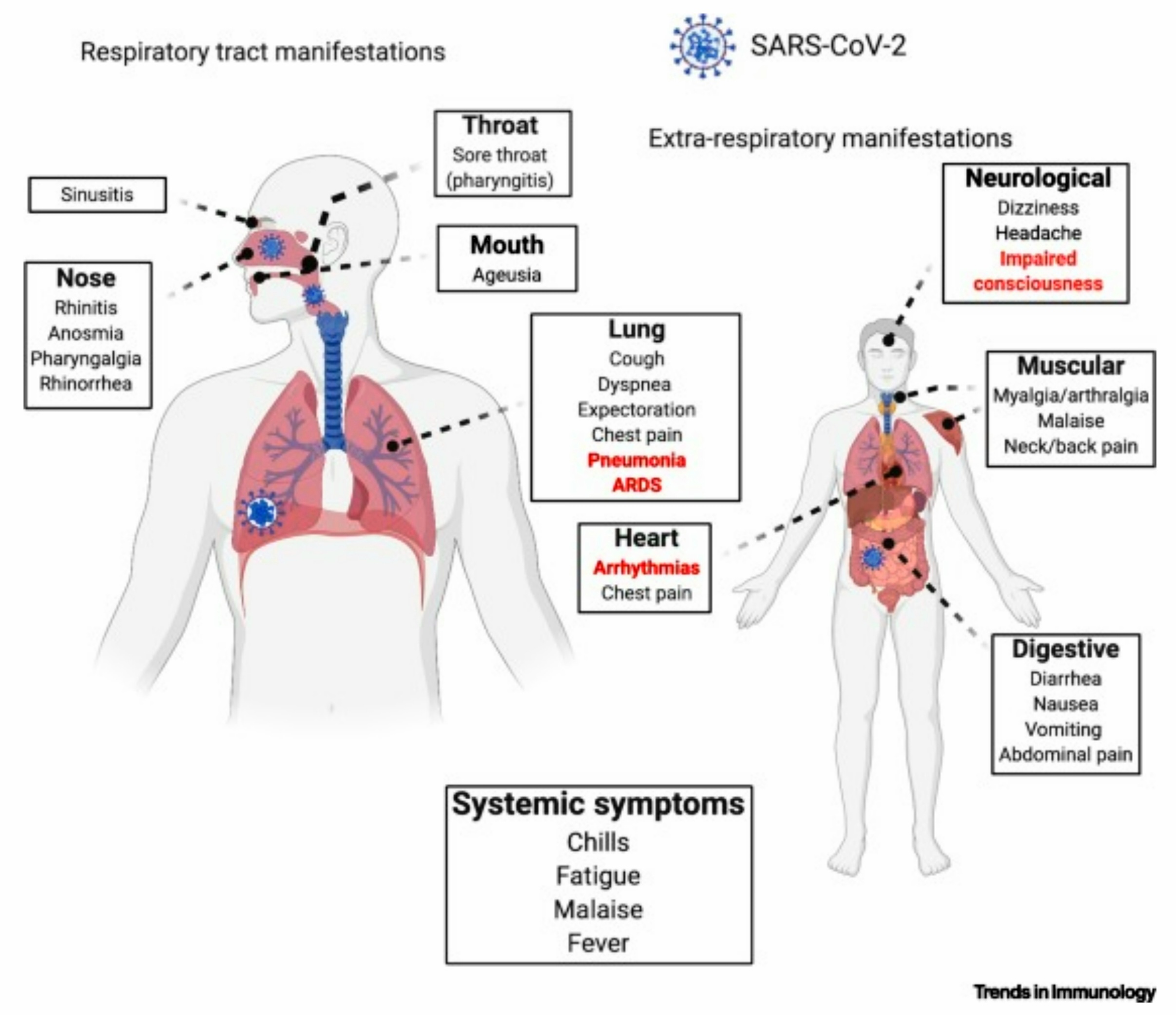
Fig 9-26 : Symptoms of Infection
Infection of SARS-CoV-2 can lead to symptoms, both common and fatal. The most common symptoms are : [56]

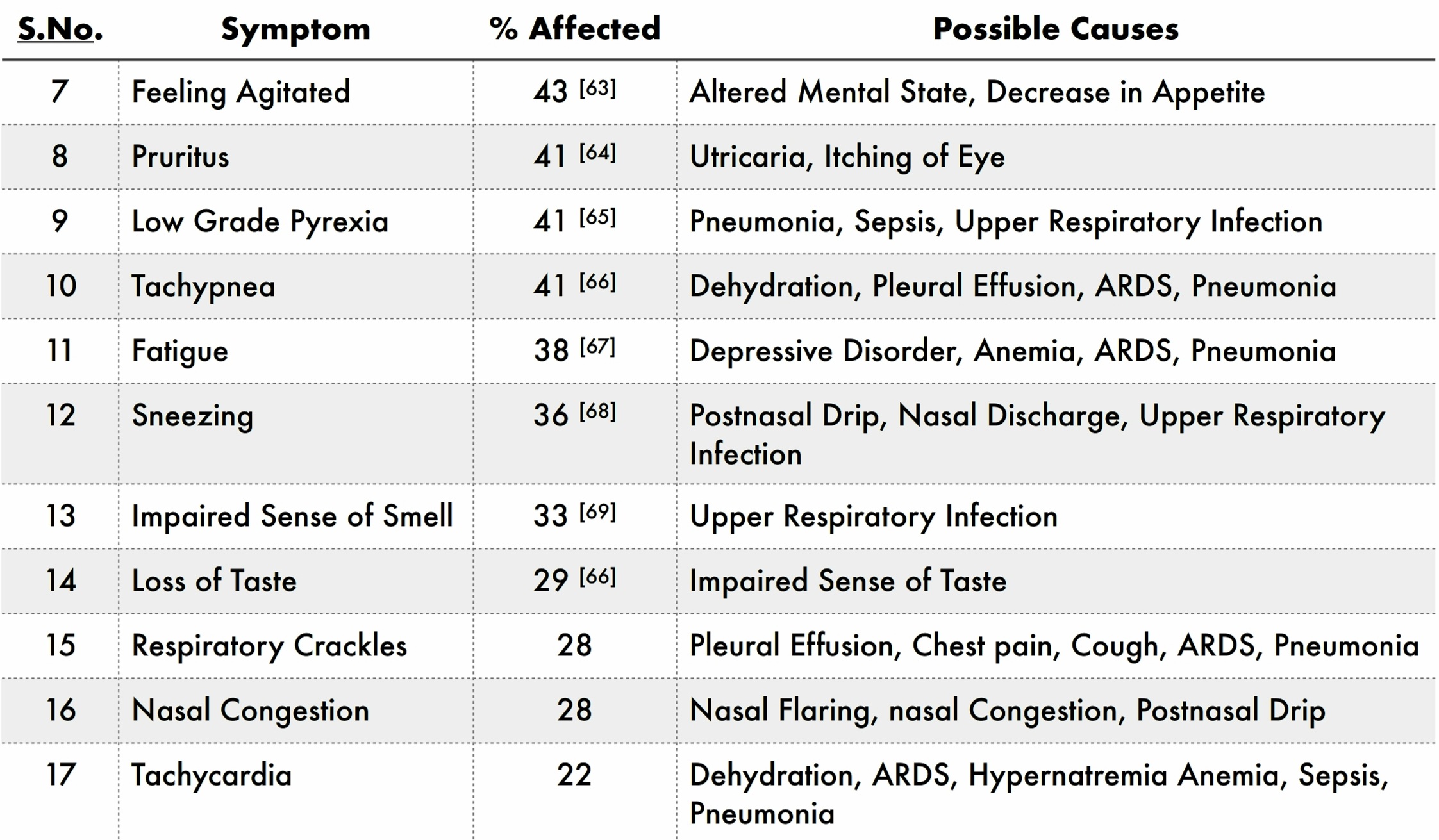
Apart from the above symptoms, a part of the population affected shows the following symptoms :
DIAGNOSIS

Fig 10-27 : RT-PCR Test
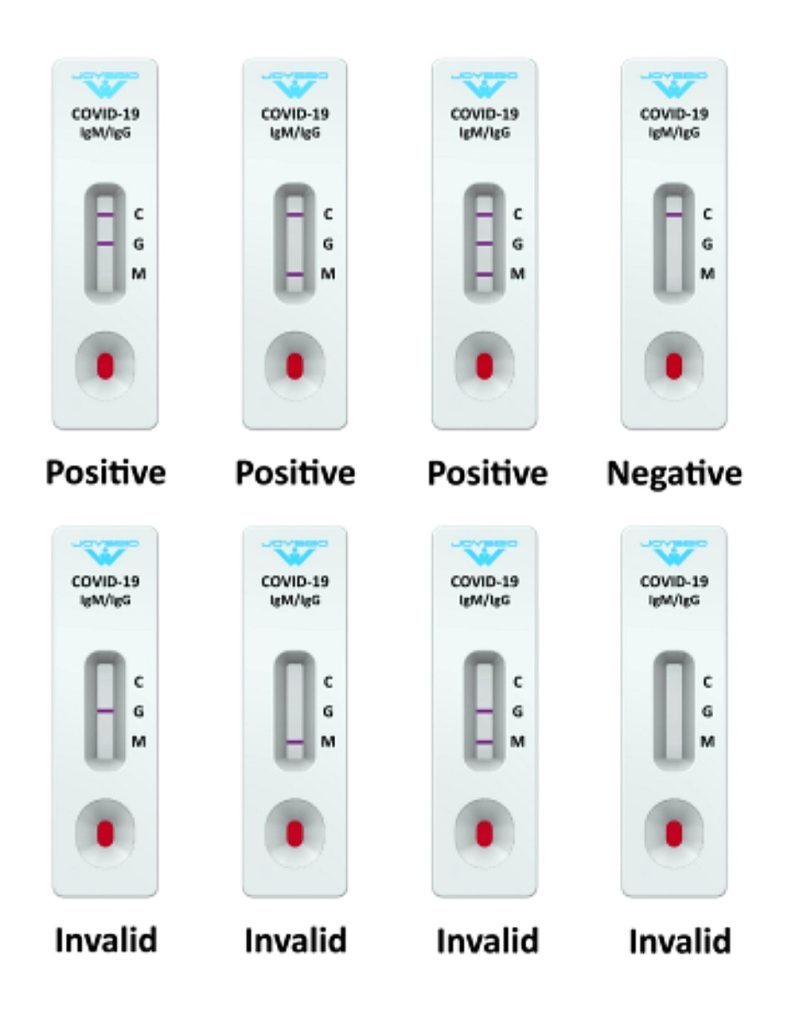
Fig 10-28 : Rapid Antibody Test
There are many different types of tests available for the fast diagnosis of COVID-19, but bellow are the four main tests available in India :
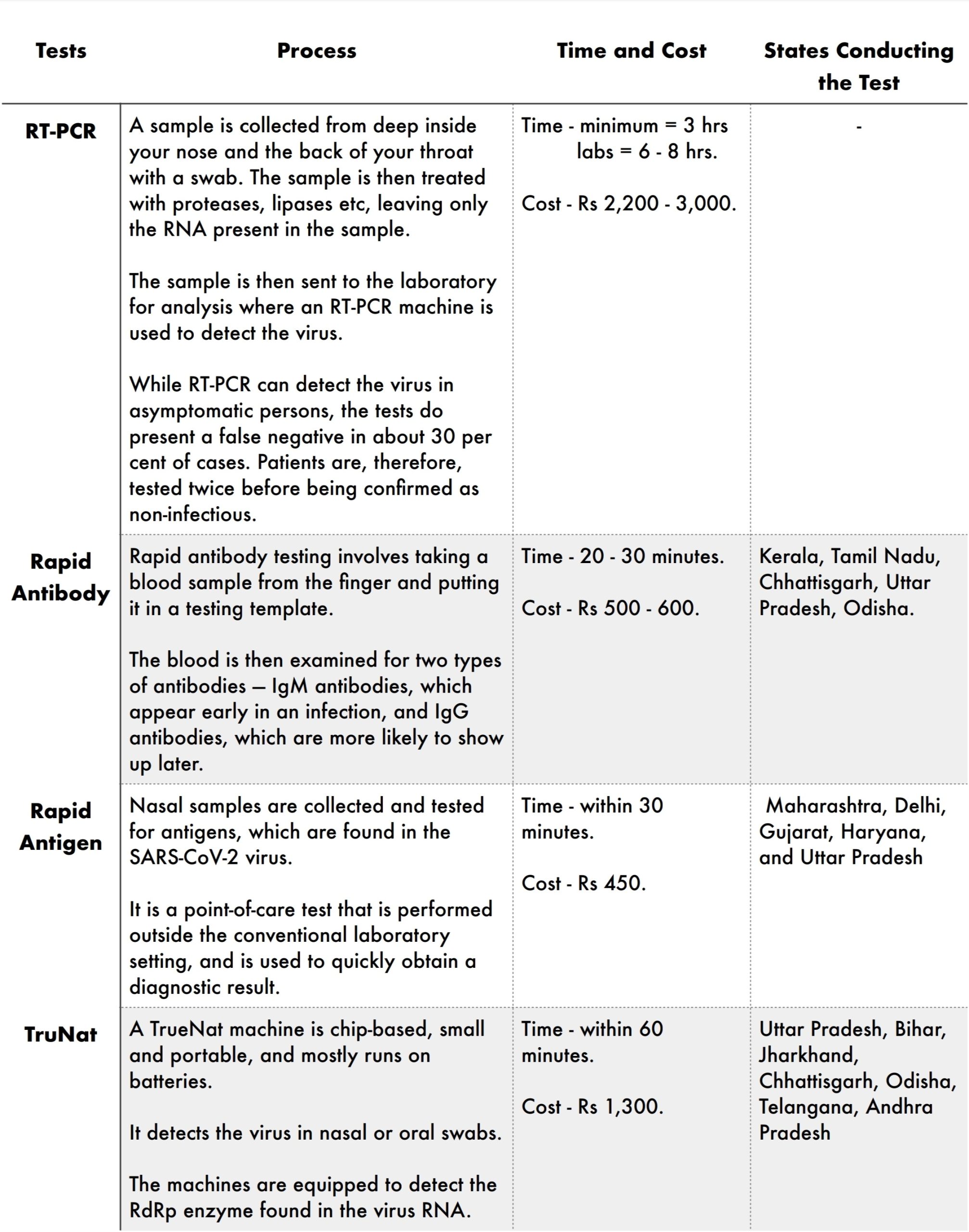
RT-PCR is the test widely used in India and around the world. The test detects the virus’ RNA, which will be present in the body before antibodies form or symptoms of the disease are present, and tells whether or not someone has the infection very early on. It proceeds by converting the RNA to DNA through a process called ‘reverse transcription’, before detecting the virus. (Fig 10-27)
Rapid Antibody tests are fast, inexpensive, and can be used to gauge the extent of infection within a community. Unlike RT-PCR, antibody tests require a blood sample to determine whether the human body has antibodies for coronavirus. Since the results are not 100 per cent reliable (they can give false positives), the ICMR has advised that if a person tests positive through a rapid test, they have to undergo a confirmatory RT-PCR test before treatment. (Fig : 10-28)

Fig 10-29 : Rapid Antigen Test Fig 10-30 : TruNat Test
Like RT-PCR, the Rapid Antigen test too, seeks to detect the virus rather than the antibodies produced by the body. It has been approved by ICMR for use in containment zones and healthcare settings. The test is reliant on the amount of virus, or the viral load, in a nasal swab. An antigen refers to any toxin in the body that triggers an immune response. (Fig : 10-29)
TruNat test, commonly used for detecting tuberculosis and HIV, works on the same principle as RT-PCR, but with a smaller kit, and produces faster results. ICMR recently approved TrueNat, manufactured by a Goa-based company, for screening and confirmation for COVID-19. (Fig : 10-30)

Fig 10-32 : Chest Imaging Fig 10-31 : CT of Chest
Other than the above tests, there are some other confirmatory tests which could be used to ensure an accurate diagnosis like:

VULNERABLE POPULATION
Severe illness means that a person with COVID-19 may require :
- hospitalization,
- intensive care, or a
- ventilator to help them breathe, or
- they may even die.
Risk for severe illness with COVID-19 increases with age, with older adults at highest risk. Certain medical conditions can also increase risk for severe illness. People at increased risk, and those who live or visit with them, need to take precautions to protect themselves from getting COVID-19.
Analysis of COVID-19 and SARS-CoV-2
AGE :

Fig 11-32 : Risk as a Function of Age
Older adults are at greater risk of requiring hospitalization or dying if they are diagnosed with COVID-19. As you get older, your risk of being hospitalized for COVID-19 increases. (Fig 11-32)
PRE-EXISTING CONDITIONS :
Adults of any age with the following conditions are at increased risk of severe illness from the virus that causes COVID-19 like,
- Cancer
- Chronic kidney disease
- COPD (chronic obstructive pulmonary disease)
- Down Syndrome
- Heart conditions (heart failure, coronary artery disease, or cardiomyopathies)
- Immunocompromised state from solid organ transplant
- Obesity (body mass index [BMI] of 30 kg/m2 or higher but < 40 kg/m2)
- Severe Obesity (BMI ≥ 40 kg/m2)
- Pregnancy
- Sickle cell disease
- Smoking
- Type 2 diabetes mellitus
Moreover, adults of any age with the following conditions might be at an increased risk for severe illness from the virus that causes COVID-19 :
- Asthma (moderate-to-severe)
- Cerebrovascular disease (affects blood vessels and blood supply to the brain)
- Cystic fibrosis
- Hypertension or high blood pressure
- Immunocompromised state from blood or bone marrow transplant; immune deficiencies; HIV; use of corticosteroids, etc
- Neurologic conditions, such as dementia
- Liver disease
- Overweight (BMI > 25 kg/m2, but < 30 kg/m2)
- Pulmonary fibrosis (having damaged or scarred lung tissues)
- Thalassemia (a type of blood disorder)
- Type 1 diabetes mellitus
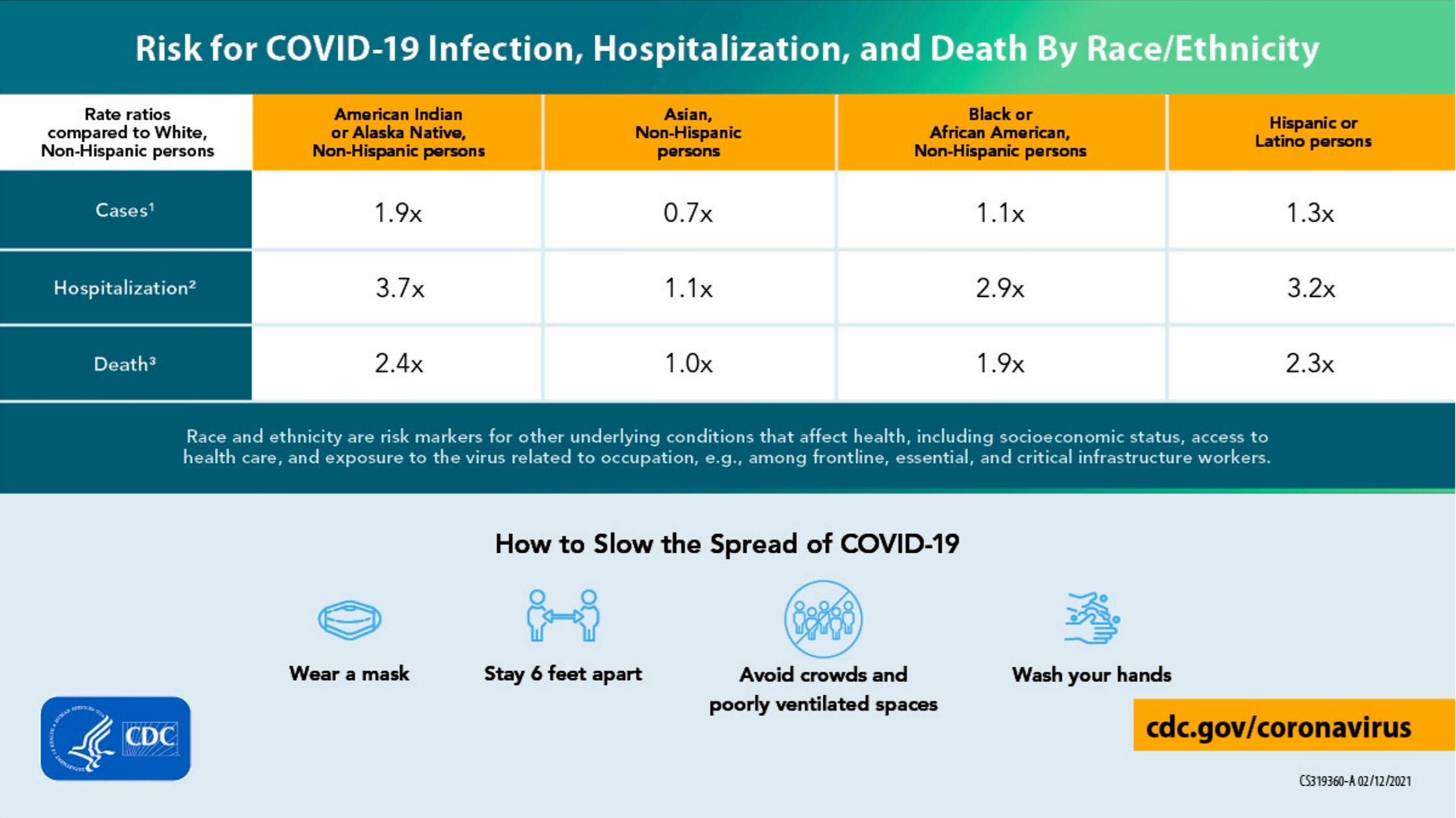
Fig 11-33 : Risk as a Function of Racial and Ethnic Minority Groups
People who also need extra precaution fall under these categories :
- People Living in Rural Communities
- People with Disabilities
- People with Developmental & Behavioral Disorders
- People Experiencing Homelessness
- Pregnant People & Breastfeeding
- Racial & Ethnic Minority Groups (Fig 11-33)
PREVENTATIVE MEASURES
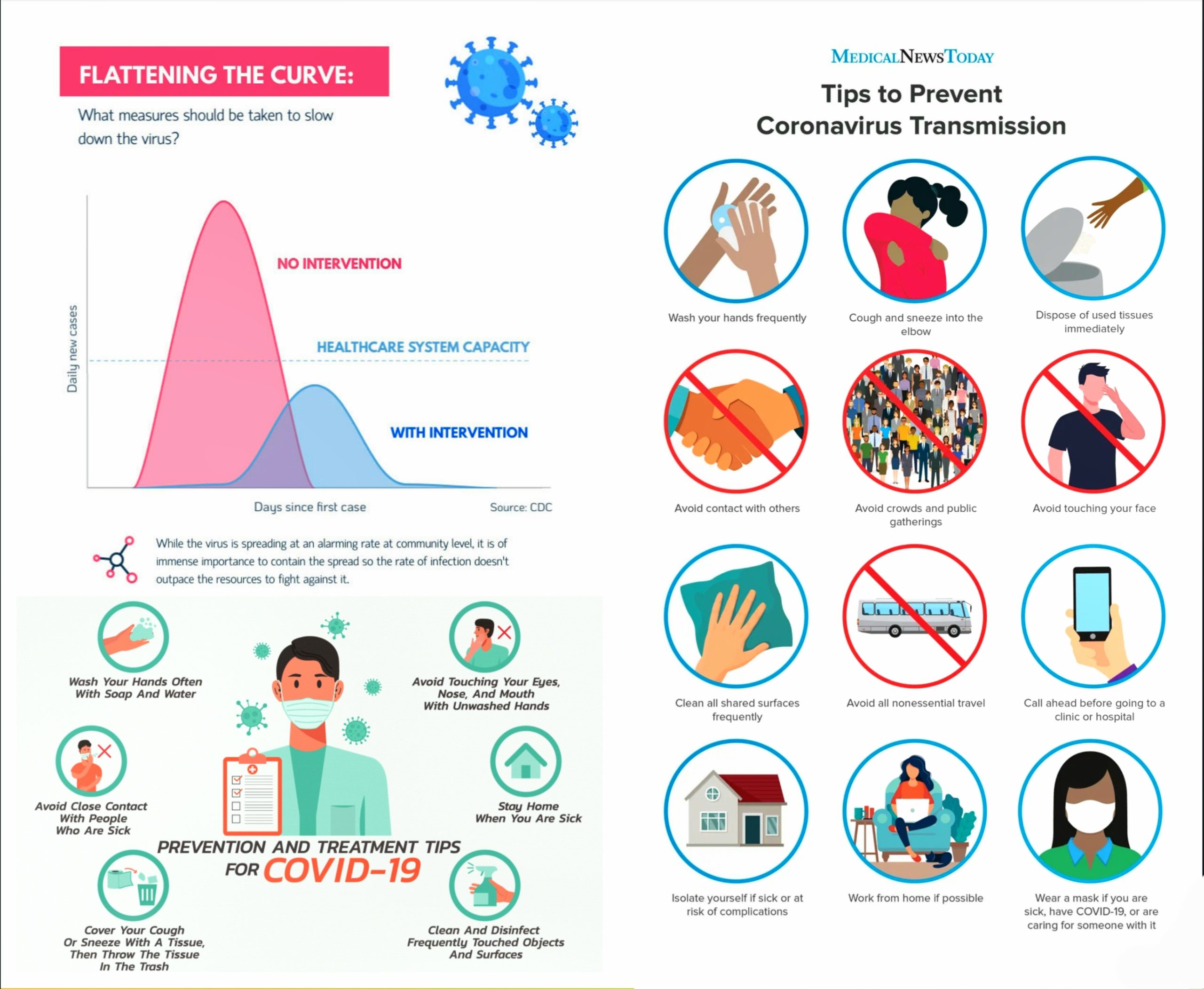
Actions to Protect Ourselves in Public :
(Fig 12.1-34)
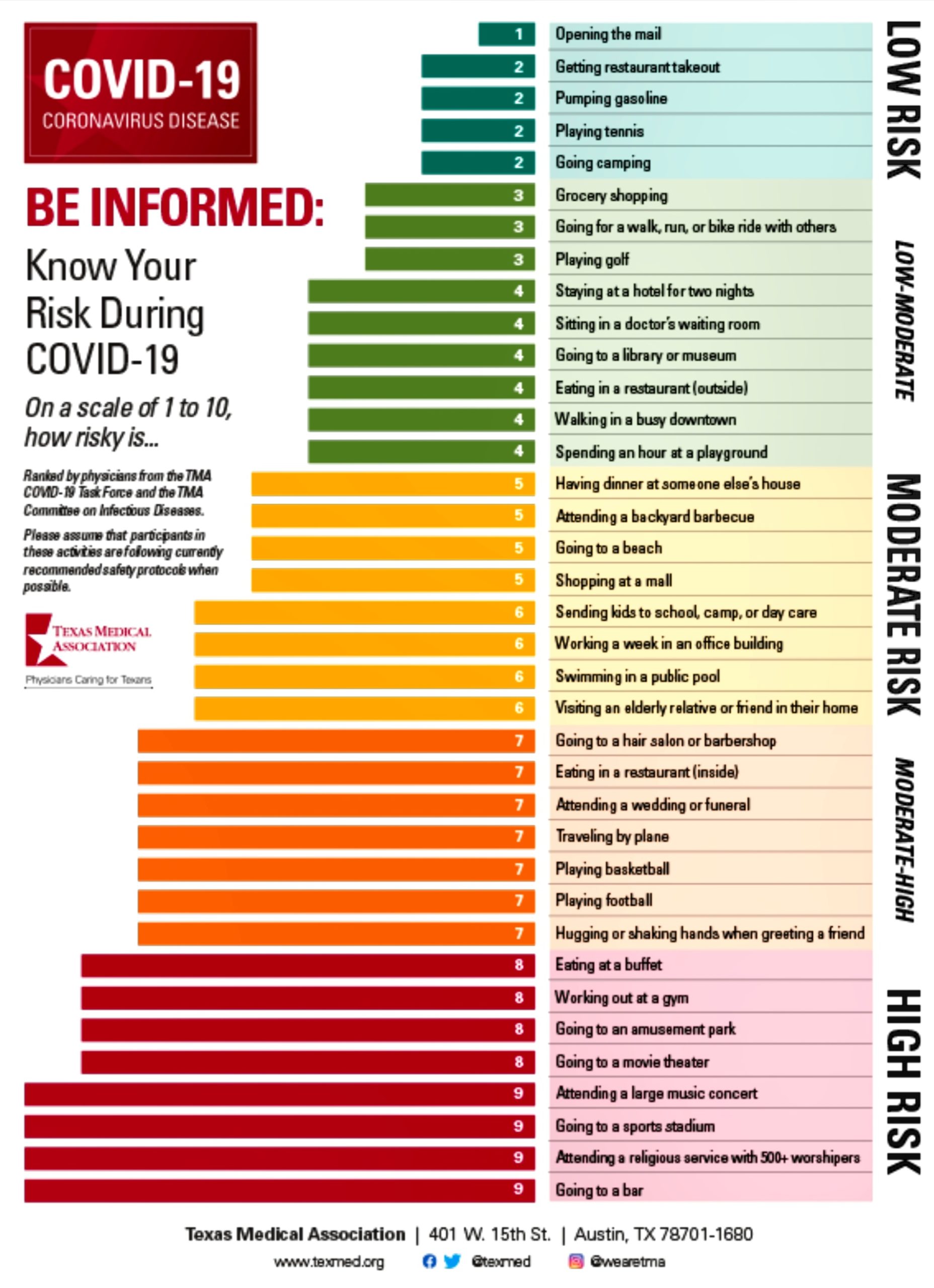
Fig 12.1-34 : Various Daily Activities and their Risks
- Avoid touching your face , especially your eyes, nose and mouth.
- Wash your hand frequently with soap and water or use alcohol-based rub.
- Seek immediate medical advice if you have fever, cough or are experiencing breathing difficulties.
- When coughing or sneezing, discard the used tissue immediately into a closed bin.
- Maintain distance of at least 1 metre from someone who is coughing or sneezing
- When coughing or sneezing, cover your mouth and nose with flexed elbow or tissue.
- If you have cough, fever, and difficulty in breathing, wear a mask correctly and seek medical advice.
Actions to Protect Ourselves at Home :
- Clean your home regularly, particularly frequently touched surfaces like door knobs, refrigerator handles, etc.
- Stay home stay safe. Stay physically fit. Exercise regulary. Eat a nutritious diet. Don’t smoke.
- Follow the Golden Rule. Wash your hands frequently with soap and water or use alcohol based hand-rub.
- Stay positive. Avoid alarmist news. Be connected to friends and family. Have a hobby.
- If any member of the household shows symptoms of Covid-19, seek medical advice and follow your local health authority's guidance.
Message for the Elderly and those having Other Diseases :
(Fig 12.3-35)
Fig 12.3-35 : General Precaution for Elderly when in Public
- Take care if you are elderly
- Avoid Exposure: Stay at home. Wash hands or use alchohol-based rub.
- Avoid touching your face.
- Eat nutritious food, exercise regularly. Stay connected with friends and family.
- Be prepared: Keep a supply of essential medicines. Keep all helpline numbers handy.
- Keep at least 1 meter apart from others.
Precautions for Pregnant and New Mothers :
(Fig 12.4-36)

Fig 12.4-36 : Precautions for Pregnant and New Mothers
- Stay at home and avoid meeting outsiders. Wash your hands with soap and water or use alcohol-based hand rub. Prepare for delivery and make arrangements to reach hospital. Its safer to deliver in a hospital, even during the COVID-19 pandemic.
- Always be alert on pregnancy related warning signs. Continue visiting your doctor or midwife for regular check ups. Immediately contact the doctor in case of symptoms like pain in abdomen, bleeding, watery discharge and severe headache. In case of an emergency visit nearest hospital.
- Wash hands before and after touching and feeding your baby. Wash clothes and sterilize all utensils & articles that come in contact with you or your baby.
- Breastfeed within 1 hour of birth and continue. It protects your baby from infections. Keep your baby close to you. Practice skin-to-skin contact (Kangaroo care) for small or preterm babies.
- If the mother is COVID positive, wear a medical mask while breastfeeding the baby. Wash your hands with soap and water or use alcohol-based hand rub before feeding. Routine clean and disinfect surfaces around you. Keep at least 1 meter distance from others.
Precautions at Work Places :
(Fig 12.5-37)

Fig 12.5-37 : Precautions at Work Places
- Keep physical distance of at least one metre.
- Wear a mask when close to others.
- Minimise the need for physical meetings by teleconferencing.
- Defer or suspend work place events and social gatherings.
- Take special care if you have pre-existing medical conditions, are pregnant or above 60 yrs in age.
- Wash your hands frequently with soap and water or use alcohol-based hand rub.
- Ensure respiratory hygiene by covering mouth and nose with flexed elbow/tissue during cough or sneeze.
- Avoid physical contact like hugging, touching, shaking hands, etc.
- If you show symptoms of COVID-19; such as sore throat, fever, difficulty in breathing, then isolate yourself, take medical advice and do not come to work.
Taking these extensive measures to prevent the spread of a pandemic is of utmost importance. Social distancing, wearing masks, and using disinfectants are a must nowadays. Although many are lathering themselves with sanitizers, people are not aware of the risks and hazards of the long term exposure to these chemical disinfectants. You can read about this in the post 'Hazards of Disinfectants' where we discuss about the disinfectants and their by-products, their toxicity and effects. While there is no need to panic, we should start moving towards a more sustainable and holistic alternative. You can read about one such alternative on 'Aloe Vera : A Sustainable Alternative'.
Analysis of COVID-19 and SARS-CoV-2
REFERENCES
Analysis of COVID-19 and SARS-CoV-2
[1] Novel Coronavirus (2019-nCoV) SITUATION REPORT - 1(21January,2020). World Health Organisation. Summary, Event highlights from 31 December 2019 to 20 January 2020.
[2] Coronavirus disease (COVID-19) Global epidemiological situation (20September,2020). World Health Organisation. Retrieved from www.who.int
[3] Tankeshar, Acharya (13June2016). Baltimore Systems of Classification of Virus. Learn Microbiology Online - Medical Microbiology Guide. Retrieved from https://microbeonline.com/baltimore-system-classifications-viruses/
[4] Shaffer, Catherine. (2019,February26). The Baltimore Classification System. News - Medical. Retrieved on December 11, 2020 from https://www.news-medical.net/life-sciences/The-Baltimore-Classification-System.aspx.
[5] Tankeshar, Acharya (13June2016). Baltimore Systems of Classification of Virus. Learn Microbiology Online - Medical Microbiology Guide. Retrieved from https://microbeonline.com/baltimore-system-lassifications-viruses/
[6] Naming the 2019 Coronavirus (11February,2020). International Committee on Taxonomy of Viruses ICTV
[7] Walls A.C., ParkY. -J., Tortorici M.A., WallA., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell.2020
[8]Schoeman D., Fielding B.C. Coronavirus envelope protein : current knowledge. Virol J. 2019.
[9] Fehr A.R., Perlman S. Springer; NewYork: 2015. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses; pp. 1–23.
[10] Huang C, Lokugamage KG, Rozovics JM, Narayanan K, Semler BL, Makino S (2011) SARS coronavirus nsp1 protein induces template-dependent endonucleolytic cleavage of mRNAs: viral mRNAs are resistant to nsp1- induced RNA cleavage. PLoS Pathog 7: e1002433
[11] Cornillez-Ty CT, Liao L, Yates JR, Kuhn P, Buchmeier MJ (2009) Severe acute respiratory syndrome coronavirus non structural protein 2 interacts with a host protein complex involved in mitochondrial biogenesis and intracellular signaling. J Virol 83:10314–10318
[12] Lei J, Kusov Y, Hilgenfeld R (2018) Nsp3 of coronaviruses: structures and functions of a large multi-domain protein. Antiviral Res 149:58–74
[13] Baez-Santos YM, St. John SE, Mesecar AD (2015) The SARS-coronavirus papain-like protease: structure, function, and inhibition by designed antiviral compounds. Antiviral Res
[14] Sakai Y, Kawachi K, Terada Y, Omori H, Matsuura Y, Kamitani W (2017) Two-amino acids change in the nsp4 of SARS coronavirus abolishes viral replication. Virology 510:165–174
[15] Tomar S, Johnston ML, St. John SE, Osswald HL, Nyalapatla PR, PaulL N, Ghosh AK, Denison MR, Mesecar AD (2015) Ligand-induced dimerization of middle east respiratory syndrome (MERS) coronavirus nsp5 protease (3CLpro) implications For nsp5 Regulation And The Development Of Antivirals. J. Biol. Chem.
[16] Cottam EM, Whelband MC, Wileman T (2014) Coronavirus NSP6 restricts autophagosome expansion. Autophagy10:1426–1441
[17] te Velthuis AJ, van de Worm SH, Snijder EJ (2012) The SARS-coronavirus nsp7+nsp8 complex is a unique multimericRNA polymerase capable of both de novo initiation and primer extension. Nucleic Acids Res 40:1737–1747
[18] Ma Y, Wu L, Shaw N, Gao Y, Wang J, Sun Y, Lou Z, Yan L, Zhang R, Rao Z (2015) Structural basis and functionalanalysis of the SARS coronavirus nsp14-nsp10 complex. Proc Natl Acad Sci USA 112:9436–9441
[19] Wang Y, Sun Y, Wu A, Xu S, Pan R, Zeng C, Jin X, Ge X, Shi Z, Ahola T, Guo D(2015) Coronavirus nsp10/nsp16methyltransferase can be targeted by nsp10-derived peptide in vitro and in vivo to reduce replication andpathogenesis. J Virol 89:8416–8427
[20] Subissi L, Posthuma CC, Collet A, Zevenhoven-Dobbe JC, Gorbalenya AE, Decroly E, Snijder EJ, Canard B, Imbert I(2014) One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase andexonuclease activities. Proc Natl Acad Sci USA
[21] Ivanov KA, Thiel V, Dobbe JC, van der Meer Y, Snijder EJ, Ziebuhr J (2004) Multiple enzymatic activities associated with severe acute respiratory syndrome coronavirus helicase.J Virol 78:5619–5632
[22] Case JB, Ashbrook AW, Dermody TS, Denison MR (2016) Mutagenesis of S-adenosyl-L-methionine-binding residues in coronavirus nsp14 N7-methyltransferase demonstrates differing requirements for genome translation and resistance to innate immunity. J Virol 90:7248–7256
[23] Hackbart M, Deng X, Baker SC (2020) Coronavirus endoribonuclease targets viral polyuridine sequences to evade activating host sensors. Proc Natl Acad Sci USA 117:8094–8103
[24] Bhardwaj K, Guarino L, Kao CC (2004) The severe acute respiratory syndrome coronavirus Nsp15 protein is an endoribonuclease that prefers manganese as a cofactor. J Virol 78:12218
[25] Decroly E, Imbert I, Coutard B, Bouvet M, Selisko B, Alvarez K, Gorbalenya AE, Snijder EJ, Canard B (2008) Coronavirus nonstructural protein 16 Is a cap-0 binding enzyme possessing (nucleoside-2’O)-methyltransferase activity. J Virol 82:8071–8084
[26] Kumar P, Gunalan V, Liu B, Chow VTK, Druce J, Birch C, Catton M, Fielding BC, Tan Y-J, Lal SK (2007) The nonstructural protein 8 (nsp8) of the SARS coronavirus interacts with its ORF6 accessory protein. Virology 366:293–303
[27] Zhao J, Falcon A, Zhou H, Netlan J, Enjuanes L, Brena PP, Perlman S (2009) Severe acute respiratory syndrome coronavirus protein 6 is required for optimal replication. J Virol 83:2368–2373
[28] Schaecher SR, Mackenzie JM, Pekosz A (2007) The ORF7b protein of severe acute respiratory syndrome coronavirus (SARS-CoV) is expressed in virus- infected cells and incorporated into SARS-CoV particles. J Virol 81:718-731
[29] P.S. Masters. The molecular biology of coronaviruses. Adv. Virus Res., 66 (2006)
[30] A. Wu, Y. Peng, B. Huang, X. Ding, X. Wang, P. Niu, J. Meng, Z. Zhu, Z. Zhang, J. Wang, J. Sheng, L. Quan, Z.
Xia, W. Tan, G. Cheng, T. Jiang. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe, 27 (2020)
[31] F. Wu, S. Zhao, B. Yu, Y.-M. Chen, W. Wang, Y. Hu, Z.-G. Song, Z.-W. Tao, J.-H. Tian, Y.-Y. Pei, M.-L. Yuan, Y.-L. Zhang, F.-H. Dai, Y. Liu, Q.-M. Wang, J.-J. Zheng, L. Xu, E.C. Holmes, Y.-Z. Zhang. Complete genome characterisation of a novel coronavirus associated with severe human respiratory disease in Wuhan, China. bioRxiv : the preprint server for biology (2020)
[32] J. Cui, F. Li, Z.L. Shi. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol., 17 (2019)
[33] One Health Basics, Zoonotic Diseases, How do germs spread between animals and people. Centre for Disease Control and Prevention (CDC). Retrieved from cdc.gov
[34] Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 2020
[35] Liu Z., Xiao X., Wei X., Li J., Yang J., Tan H., Zhu J., Zhang Q., Wu J., Liu L. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J Med Virol. 2020
[36] R. Lu, X. Zhao, J. Li, P. Niu, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet, 395 (2020), pp. 565-574
[37] L.-L. Ren, Y.-M. Wang, Z.-Q. Wu, Z.-C. Xiang, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl)., 133 (2020), pp. 1015-1024
[38] D.R. Murdoch, N.P. French. COVID-19: another infectious disease emerging at the animal-human interface. N Z Med J, 133 (2020), pp. 12-15
[39] OIE. Animal and environmental investigations to identify the zoonotic source of the COVID-19 Virus (2020)
[40] S. Mallapatty. Coronavirus can infect cats — dogs, not so much. Nature (2020)
[41] M. Chini. Coronavirus: Belgian cat infected by owner. The Brussels Times (2020)
[42] WCS Newsroom. A tiger at bronx zoo tests positive for COVID-19; the tiger and the zoo's other cats are doing well at this time (2020)
[43] P. Sun, S. Qie, Z. Liu, J. Ren, J. Jianing Xi. Clinical characteristics of 50466 patients with 2019-CoV infection. medRxiv (2020)
[44] C. Salata, A. Calistri, C. Parolin, G. Palù. Coronaviruses: a paradigm of new emerging zoonotic diseases. Pathogens and Disease, 77 (2020)
[45] Y. Wang, M. Liu, J. Gao. Enhanced receptor binding of SARS-CoV-2 through networks of hydrogen-bonding and hydrophobic interactions. Proc Natl Acad Sci Unit States Am, 117 (2020)
[46] W. Ji, W. Wang, X. Zhao, J. Zai, X. Li. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol, 92 (2020)
[47] X. Li, J. Zai, Q. Zhao, Q. Nie, Y. Li, B.T. Foley, A. Chaillon. Evolutionary history,potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol, 92 (2020)
[48] T. Zhang, Q. Wu, Z. Zhang. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol, 30 (2020)
[49] Li Huanjie, Wang Yangyang, Ji Mingyu, Pei Fengyan, Zhao Qianqian, Zhou Yunying, Hong Yatian, Han Shuyi, Wang Jun, Wang Qingxi, Li Qiang, Wang Yunshan. Transmission Routes Analysis of SARS-CoV-2: A Systematic Reviewand Case Report. Frontiers in Cell and Developmental Biology (2020).
[50] Fears AC, Klimstra WB, Duprex P, Hartman A, Weaver SC, Plante KS, et al. Persistence of severe acute respiratory syndrome coronavirus 2 in aerosol suspensions. Emerg Infect Dis. 2020 Sep;29(9). Available from: https://doi.org/10.3201/eid2609.201806.
[51] Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen H-L, et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020. Available from: https://doi.org/10.1016/S2666-5247(20)30003-3.
[52] Pastorino B, Touret F, Gilles M, Lamballerie Xd, Charrel R. Prolonged infectivity of SARS-CoV-2 in fomites. Emerg Infect Dis. 2020 Sep;26(9). Available from https://wwwnc.cdc.gov/eid/article/26/9/20-1788_article
[53] Chen, N., Zhou, M., Dong, X., Qu, J., Gong, F., Han, Y., et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513
[54] Wong, C. K., Lam, C. W., Wu, A. K., Ip, W. K., Lee, N. L., Chan, I. H., et al. (2004). Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 136, 95–103
[55] Wu, C., Hu, X., Song, J., Du, C., Xu, J., Yang, D., et al. (2020). Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19).
[56] Coronavirus (COVID-19) - Updated Clinical Knowledge. Retrieved from https://coronavirus.kahun.com/
[57] Chen H, Li Q, Liu R, Wang D, Xu H, Yang M, et al. Epidemiological characteristics and clinical features of 32 critical and 67 noncritical cases of COVID-19 in Chengdu. J Clin Virol [Internet]. 2020 Apr 10
[58] Fan C-Y, He C, Miao Q, Wang A-L, Xia W-G, Xu B, et al. Suppressed T cell-mediated immunity in patients with COVID-19: A clinical retrospective study in Wuhan, China. J Infect [Internet]. 2020 Apr 18
[59] Covassin N, Fan Z, Gao W, Kara T, Li G, Singh P, et al. Association Between Hypoxemia and Mortality in Patients With COVID-19. Mayo Clin Proc [Internet]. 2020 Apr 11
[60] Fortune B, Hajifathalian K, Krisko T, Kumar S, Mehta A, Schwartz R, et al. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology [Internet].2020 May 8
[61] Cheng Z, Hu B, Hu C, Li Y, Liu X, Peng Z, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA [Internet]. 2020 Feb 7
[62] Arora S, Karamanis D, Kokkinidis DG, Li W, Mantzoros CS, Ognibene J, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism [Internet]. 2020 May 16
[63] Carey I, Edmonds P, Etkind SN, Higginson IJ, Lovell N, Maddocks M, et al. Characteristics, symptom management and outcomes of 101 patients with COVID-19 referred for hospital palliative care. J Pain Symptom Manage [Internet]. 2020 Apr 20
[64] Berti E, Calzavara-Pinton P, Caputo V, Cusini M, Fabbrocini G, Genovese G, et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol [Internet]. 2020 Apr 16
[65] Fang Y, Huang M, Li K, Li W, Li S, Liao Y, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur Radiol [Internet]. 2020 Mar 25
[66] Biagi A, Comastri G, Gandolfi S, Malagoli A, Rossi L, Sticozzi C, et al. Clinical and epidemiological characteristics of 320 deceased Covid-19 patients in an Italian Province: a retrospective observational study. J Med Virol [Internet]. 2020 Jun 9
[67] Cao J, Cheng W, Hu X, Liu Y-K, Liu Q, Tu W-J, et al. Clinical Features and Short-term Outcomes of 102 Patients with Corona Virus Disease 2019 in Wuhan, China. Clin Infect Dis [Internet]. 2020 Apr 2
[68] Augustin M, Dewald F, Heindl LM, Klein F, Lehmann C, Loreck N, et al. Olfactory and Gustatory Dysfunction in Coronavirus Disease 19 (COVID-19). Clin Infect Dis [Internet]. 2020 May 1
[67] Andreoni M, Balbi O, Biagio Mercuri N, Cesta N, Genga Mina G, Iannetta M, et al. Subjective neurological symptoms frequently occur in patients with SARS-CoV2 infection. Brain Behav Immun [Internet]. 2020 May 13
[68] Boone CE, DeConde AS, Faraji F, Prajapati DP, Yan CH. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol [Internet]. 2020 Apr 12
[69] Cai J, Chang H, Ge Y, Jia R, Li J, Lin D, et al. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis [Internet]. 2020 Feb 28
[70] Hoffmann, M., Kleine-Weber, H., Schroeder, S., Kruger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271.e8–280.e8.
[71] Bestle D, Heindl MR, Limbu H et al. TMPRSS2 and furin are both essential for proteolytic activation and spread of SARS-CoV-2 in human airway epithelial cells and provide promising drug targets. bioRxiv doi: https://doi.org/10.1101/2020.04.15.042085 (2020).
[72] Yuan M, Wu MC, Zhu X, et al. A highly conserved cryptic epitope in the receptor-binding domains of SARS-CoV-2 and SARS-CoV. Science 10.1126/science.abb7269 (2020).
[73] Gui M, Song W, Zhou H, et al. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res. 27, 119–129 (2017).
[74] Walls AC, Xiong X, Park YJ, et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell 176, 1026–1039.e15 (2019).
[75] Walls AC, Tortorici MA, Snijder J, et al. Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proc. Natl. Acad. Sci. U.S.A. 114, 11157–11162 (2017).
[76] Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 36(6483), 1260-1263 (2020).
[77] Denison, M. R. & Perlman, S. Translation and processing of mouse hepatitis virus virion RNA in a cell-free system. J. Virol. 60, 12–18 (1986).
[78] Kamitani, W. et al. Severe acute respiratory syndrome coronavirus nsp1 protein suppresses host gene expression by promoting host mRNA degradation. Proc. Natl Acad. Sci. USA 103,12885–12890 (2006).
[79] Thoms, M. et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science https://doi.org/10.1126/science.abc8665 (2020).
[80] Schubert, K. et al. SARS-CoV-2 Nsp1 binds the ribosomal mRNA channel to inhibit translation. Nat. Struct. Mol. Biol. https://doi.org/10.1038/s41594-020-0511-8 (2020).
[81] Sims, A. C., Ostermann, J. & Denison, M. R. Mouse hepatitis virus replicase proteins associate with two distinct populations of intracellular membranes. J. Virol. 74, 5647–5654 (2000).
[82] Snijder, E. J., Decroly, E. & Ziebuhr, J. The nonstructural proteins directing coronavirus RNA synthesis and processing. Adv. Virus Res. 96, 59–126 (2016).
[83] V’kovski, P. et al. Determination of host proteins composing the microenvironment of coronavirus replicase complexes by proximity-labeling. eLife 8, e42037 (2019).
[84] Perlman, S. & Netland, J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 7, 439–450 (2009).
[85] Gao, Y. et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 368, 779–782 (2020).
[86] Tvarogová, J. et al. Identification and characterization of a human coronavirus 229E Nonstructural protein 8-associated RNA 3′-terminal adenylyltransferase activity. J. Virol. https://doi.org/10.1128/JVI.00291-19 (2019).
[87] Eckerle, L. D., Lu, X., Sperry, S. M., Choi, L. & Denison, M. R. High fidelity of murine hepatitis virus replication is decreased in nsp14 exoribonuclease mutants. J. Virol. 81, 12135–12144 (2007).
[88] Zhang, L. et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved a-ketoamide inhibitors. Science 368, 409–412 (2020).
[89] Anand, K., Ziebuhr, J., Wadhwani, P., Mesters, J. R. & Hilgenfeld, R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science 300, 1763–1767 (2003).
[90] Jin, Z. et al. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature 582, 289–293 (2020).
[91] Sawicki, S. G. & Sawicki, D. L. Coronaviruses use discontinuous extension for synthesis of subgenome-length negative strands. Adv. Exp. Med. Biol. 380, 499–506 (1995).
[92] Sola, I., Almazán, F., Zúñiga, S. & Enjuanes, L. Continuous and discontinuous RNA synthesis in coronaviruses. Annu. Rev. Virol. 2, 265–288 (2015).
[93] Kim, D. et al. The architecture of SARS-CoV-2 transcriptome. Cell 181, 914–921 (2020).
[94] Viehweger, A. et al. Direct RNA nanopore sequencing of full-length coronavirus genomes provides novel insights into structural variants and enables modification analysis. Genome Res. 29, 1545–1554 (2019).
[95] Di, H., McIntyre, A. A. & Brinton, M. A. New insights about the regulation of Nidovirus subgenomic mRNA synthesis. Virology 517, 38–43 (2018).
[96] Stertz, S. et al. The intracellular sites of early replication and budding of SARS-coronavirus. Virology 361, 304–315 (2007).
[97] de Haan, C. A. & Rottier, P. J. Molecular interactions in the assembly of coronaviruses. Adv. Virus Res. 64, 165–230 (2005).
[98] Klein, S. et al. SARS-CoV-2 structure and replication characterized by in situ cryo-electron tomography. Preprint at bioRxiv https://doi.org/10.1101/2020.06.23.167064 (2020).
[99] Davidson, A. D. et al. Characterisation of the transcriptome and proteome of SARS-CoV-2 reveals a cell passage induced in-frame deletion of the furin-like cleavage site from the spike glycoprotein. Genome Med. 12, 68 (2020).
[100] Chen L, Fang Z, Guo D, Huang H, Li C, Wu J, et al. Chest CT Findings in Patients with Corona Virus Disease 2019 and its Relationship with Clinical Features. Invest Radiol [Internet]. 2020 Feb 21
[101] Bang JH, Chin BS, Choi JP, Chung KH, Kang CK, Kang YM, et al. Clinical Course and Outcomes of Patients with Severe Acute Respiratory Syndrome Coronavirus 2 Infection: a Preliminary Report of the First 28 Patients from the Korean Cohort Study on COVID-19. J Korean Med Sci [Internet]. 2020 Apr 6
[102] Du W, Li Q, Wang H, Yu J, Zhang X, Zhang S, et al. Clinical characteristics of COVID-19 in children compared with adults in Shandong Province, China. Infection [Internet]. 2020 Apr 16
[103] Liu J, Xie X, Yu Q, Zhao W, Zhong Z. CT Scans of Patients with 2019 Novel Coronavirus (COVID-19) Pneumonia. Theranostics [Internet]. 2020 Mar 15
[104] Ji P, Li H, Li B, Pang J, Zhang J, Zhong Z, et al. CT imaging features of 4,121 patients with COVID-19: a meta-analysis. J Med Virol [Internet]. 2020 Apr 21
[105] Chen D, Hong L, Luo Y, Qiu H, Song Q, Wu J. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis [Internet]. 2020 Mar25
[106] Cao Q, Chen J, Guo Y, Han T, He S, Hou C, et al. Clinical and Autoimmune Characteristics of Severe and Critical Cases with COVID-19. Clin Transl Sci [Internet]. 2020 Apr 21
[107] He C, Ji P, Li H, Pang J, Zhang J, Zhao C, et al. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol [Internet]. 2020 Apr 15
[108] Cao B, Chen H, Du R, Fan G, Gu X, Guan L, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet [Internet]. 2020 Mar 11
Join Weekly Health Newsletter
Every week, I will share with you information about Natural Health, Diet, Exercise, Yoga & Wellness.

0 comments