Disinfectants have become a necessary evil in today's world. Although they are effective in cleaning, they affect us and our surrounding in ways many don't even know. Hazards of disinfectants many be acute [inflammation, uneasiness] or may cause irreversible disabilities [lung damage, blindness]. And let's not forget what might happen to a child, a pregnant woman or an elder.
This post is a part of my chemistry project for the last year of my highschool. The project was a practical to find whether a plant [Aloe vera] could be an effective disinfectant. Its foundation was built on the fact that all the harsh chemicals we use as disinfectants affect us in ways we might not think were possible. Adding to that, we should work on finding sustainable alternatives due to the risks involved and I wanted to explore one such possibility.
Join Weekly Health Newsletter
Every week, I will share with you information about Natural Health, Diet, Exercise, Yoga & Wellness.

Fig 1-1: Different Household Disinfectants
According to the National Cancer Institute, any substance or process that is used primarily on non-living objects to kill germs, such as viruses, bacteria, and other microorganisms that can cause infection and disease is called as a disinfectant. [1]
Most disinfectants are harsh chemicals but sometimes heat or radiation may be used. (Fig 1-1)
Other subtances do exist which give the same result but at different locations. Antibiotics work inside our body and antiseptics work on living surfaces.
Disinfectants too can be classified on the basis of their location of use :
- Medical grade
- Instrument grade
- Low, Intermediate and High level [due to their effect on different microorganisms]
- Sterilant
- Household grade
- Commercial grade
and their chemical composition :
- Oxidising Agent
- Peroxides
- Chlorine and its derivatives
- Heavy metals
- Organic [Alcohol, Aldehyde, Phenol, Ammonium salt]
Although effective in completing their role, there are hazards of disinfectants that the user should be aware of.
DIRECT HARMFUL EFFECTS OF SYNTHETIC DISINFECTANTS

Fig 2-2: Spraying Disinfectants on People
Chlorine (Cl2) has been commonly used throughout the world as a chemical disinfectant, with the purpose of being a principal barrier to microbial contaminants in drinking-water. The evident biocidal attributes of chlorine have been somewhat offset by the formation of disinfectant by-products (DBPs) of public health concern during the chlorination process. Consequently, alternative chemical disinfectants, such as ozone (O3), chlorine dioxide (ClO2) and chloramines (NH2Cl, monochloramine), are increasingly being used; however, each has been shown to form its own set of DBPs. [2]
Chlorine gas, chloramine and chlorine dioxide are powerful respiratory irritants. Bleach (chemically, sodium hypochlorite NaOCl) has been observed to be used in human poisoning. [3]
Improper cleaning of surfaces applied with disinfectants can contain residual disinfectants. They will react easily with organic compounds found in saliva and stomach content, resulting in the formation of by-products.[4] These foreign compounds can be absorbed by the body, which can affect the pHof the blood, disturb the inner linings of organs, or even necroses some tissues.
On 18th April 2020, the Union Health Ministry of India issued an advisory against spraying of disinfectant on people for COVID-19 management. They stated "Spraying of individuals or groups is not recommended under any circumstances. Spraying an individual or group with chemical disinfectants is physically and psychologically harmful”.[5] (Fig 2-2)

Fig 2.1-3: Igniting chlorine tanks in WWII
![Fig 2.1-4: Dermatitis [Inflammation of skin] due to chlorine exposure Fig 2.1-4: Dermatitis [Inflammation of skin] due to chlorine exposure](https://devadityatomar.com/wp-content/uploads/Screenshot_20221010_175810_OneDrive-1024x855.jpg)
Fig 2.1-4: Dermatitis [Inflammation of skin] due to chlorine exposure
EFFECTS OF CHLORINE :
Chlorine has long been recognised as a lung irritant and its amount is negligible in drinking water, but it surely has some occupational hazards. Similarly, Sodium hypochlorite (NaOCl) or calcium hypochlorite (Ca(OCl)2) are also extensively used as disinfectants, for they are highly caustic. This too leads to a clear concern for occupational exposure.
Some major sources of toxicity in humans are by exposure to chlorine and hypochlorite solutions, chlorine gas, and intake of sodium hypochlorite, marketed as bleach.
Yarington (1970) demonstrated that instillation of bleach (NaOCl) into the oesophagus of dogs causes irritation. The minimal exposure of 5 mins with 10 ml commercial bleach produced irritation. (Fig 2.1-4) [6]
The irritating effects of chlorine have been well observed and recorded, thanks to the chemical warfare during World War I.(Fig 2.1-3) [7] In the follow-up of survivors of gas chambers in concentration camps, no permanent lung damage was observed, however breath sounds indicated bronchitis and limited chest and diaphragmatic movement, even emphysema.
EFFECTS OF CHLORAMINE :
Chloramine is primarily used as a residual disinfectant, and chloramines (as a group) are a potent respiratory irritant.
Eaton et al. (1973) concluded with his research that chloramine is capable of inducing methemoglobinemia at low concentrations when there is a large reservoir of chloramine. This is a different exposure pattern from that of normal mammals, as they consume small volumes of water in comparison to the volume of red blood cells exposed to the water. [8]
The primary harmful effects have been observed in human poisoned by chloramine formed when household bleach (NaOCl) was mixed with ammonia (NH3) for use as a cleaning solution.
FORMATION OF DISINFECTANT BY-PRODUCTS
Disinfectant By-Products (DBPs) are formed upon the reaction of chemical disinfectants with DBP precursors. Natural organic matter (NOM) acts as the organic precursor, whereas bromide ion (Br-) serves as the inorganic precursor. DBP formation is influenced by water quality (e.g., bromide, pH, temperature, etc.) and treatment conditions (e.g., disinfectant dose, contact time, removal of NOM before the point of disinfectant application, prior addition of disinfectant, etc.). [9]
CHLORINE BY-PRODUCTS :
Chlorine is used in the form of gaseous chlorine or hypochlorite (OCl-). As an oxidizing agent, chlorine reacts with a wide variety of compounds, in particular those that are considered reducing agents (hydrogen sulfide [H2S], manganese(II), iron(II), sulfite [SO32-], Br-, iodide [I-], nitrite.

Eq 3.1-1

Eq 3.1-2
Chlorine gas hydrolyses in water almost completely to form hypochlorous acid (HOCl) (Eq 3.1-1). The hypochlorous acid dissociates into hydrogen ions (H+) and hypochlorite ions in the reversible reaction (Eq 3.1-2).
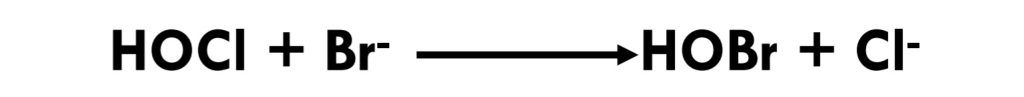
Eq 3.1-3
Eq 3.1-4
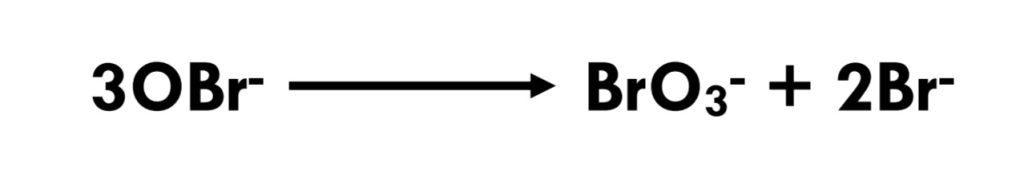
Chlorine in the form of hypochlorous acid/hypochlorite ion (HOCl/OCl-) reacts with bromide ion, oxidizing it to hypobromous acid/hypobromite ion (HOBr/OBr-).(Eq 3.1-3) Hypobromous acid is a weak acid (pKa = 8.7); like hypochlorite, hypobromite is metastable. In alkaline solution, it decomposes to give bromate and bromide.(Eq 3.1-4)
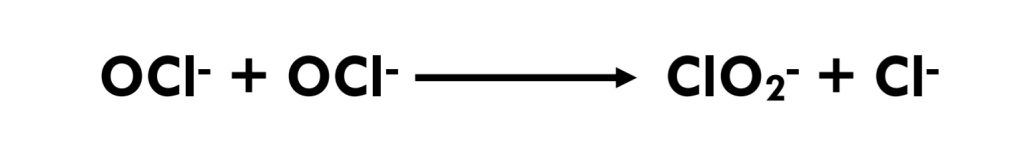
Eq 3.1-5

Eq 3.1-6
Another reaction that occurs with chlorine is the formation of chlorate (ClO3-) in concentrated hypochlorite solutions.(Eq 3.1-5 and Eq 3.1-6)
Hypochlorous acid (a more powerful oxidant) and hypobromous acid (a more effective halogenating agent) react collectively with NOM to form chlorine DBPs, including trihalomethanes (THMs), haloacetic acids (HAAs), haloacetonitriles (HANs), haloketones, chloral hydrate and chloropicrin. [10]
CHLORINE DIOXIDE BY-PRODUCTS :
Eq 3.2-7
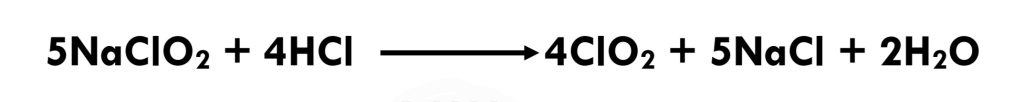
Eq 3.2-8
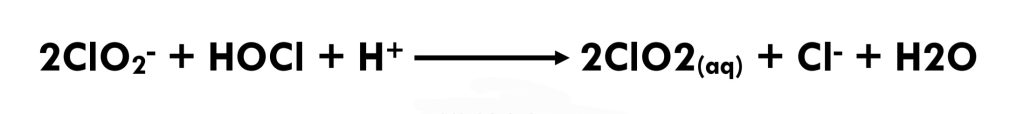
Chlorine dioxide is generally produced by reacting aqueous (sodium) chlorite with chlorine.(Eq 3.2-7) An alternative approach to chlorine dioxide generation is with hydrochloric acid (HCl), a process that results in less chlorate during production.[11] (Eq 3.2-8)
Chlorine dioxide is one of the few compounds that exists almost entirely as monomeric free radicals. Concentrated chlorine dioxide vapour is potentially explosive because of which it must be manufactured at the point of use. Chlorine dioxide in water does not hydrolyse to any appreciable extent. Neutral or acidic dilute aqueous solutions are quite stable if kept cool, well-sealed and protected from sunlight.
Chlorine dioxide represents an oxidation state (+4) intermediate between those of chlorite (+3) and chlorate (+5). No acid or ion of the same degree of oxidation is known.
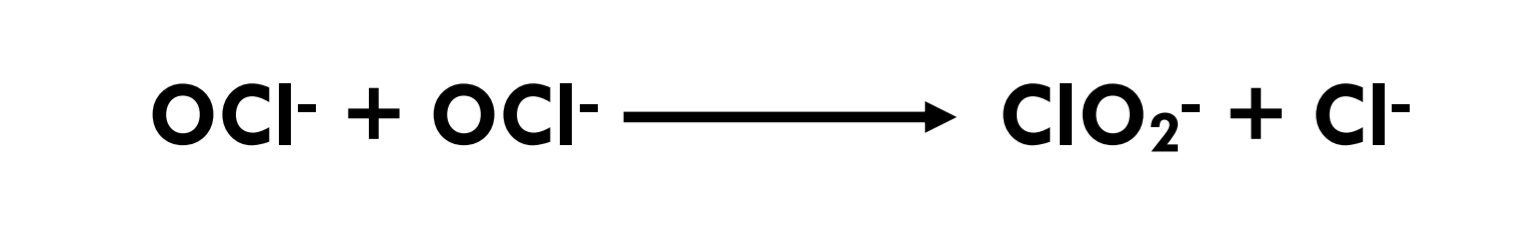
Eq 3.1-5
Chlorine dioxide is a powerful oxidizing agent that can decompose to chlorite; in the absence of oxidizable substances and in the presence of alkali, it dissolves in water, decomposing with the slow formation of chlorite and chlorate [12] (Eq 3.2-9) When chlorine dioxide reacts with aqueous contaminants, it is usually reduced to chlorite ion. The corresponding electron transfer reactions are comparable to those occurring when singlet oxygen acts as an oxidant (Tratnyek & Hoigne, 1994).[13]
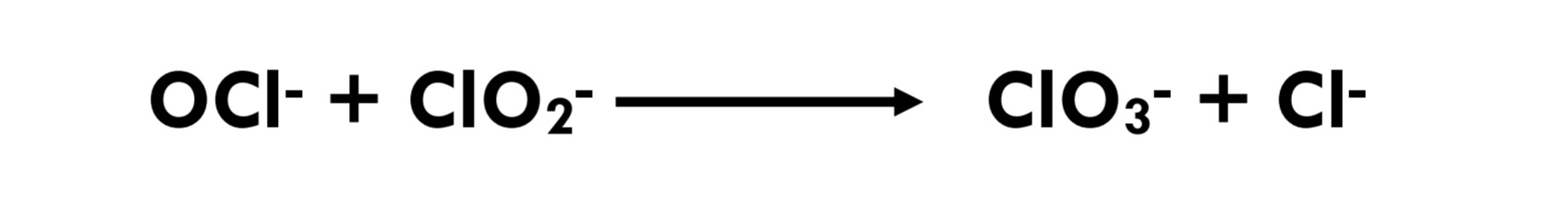
Eq 3.1-6
Bromide (in the absence of sunlight) is not oxidized by chlorine dioxide. (Eq 3.2-10) Therefore, water treatment with chlorine dioxide will not transform bromide ion into hypobromite and will not give rise to the formation of bromoform (CHBr3) or bromate. This is an important difference between the use of chlorine dioxide as an oxidant and the use of chlorine or ozone as an oxidant. Unlike the other disinfectants, the major chlorine dioxide DBPs are derived from decomposition of the disinfectant as opposed to reaction with precursors.[12]
OZONE BY-PRODUCTS :
Ozone is a strong oxidizing agent. Oxidation reactions initiated by ozone in water are generally rather complex; in water, only part of the ozone reacts directly with dissolved solutes. Another part may decompose before reaction. Such decomposition is catalysed by hydroxide ions (OH-) and other solutes.

Eq 3.3-11
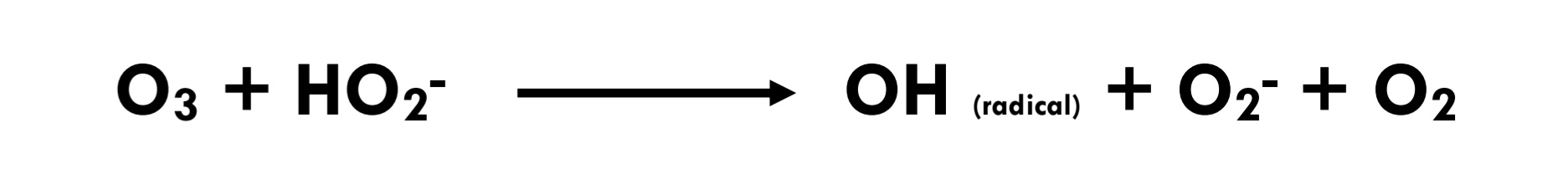
Eq 3.3-12
Highly reactive secondary oxidants, such as hydroxyl radicals (OH-), are thereby formed. (Eq 3.3-11) These radicals and their reaction products can additionally accelerate the decomposition of ozone. (Eq 3.3-12) Consequently, radical-type chain reactions may occur, which consume ozone concurrently with the direct reaction of ozone with dissolved organic material.
Many oxidative applications of ozone have been developed, including disinfection, control of algae, removal of tastes and odours, removal of colour, removal of iron and manganese, microflocculation, removal of turbidity, removal of organics by oxidation of phenols, detergents and some pesticides, and control of halogenated organic compounds.

Eq 3.3-13

Eq 3.3-14
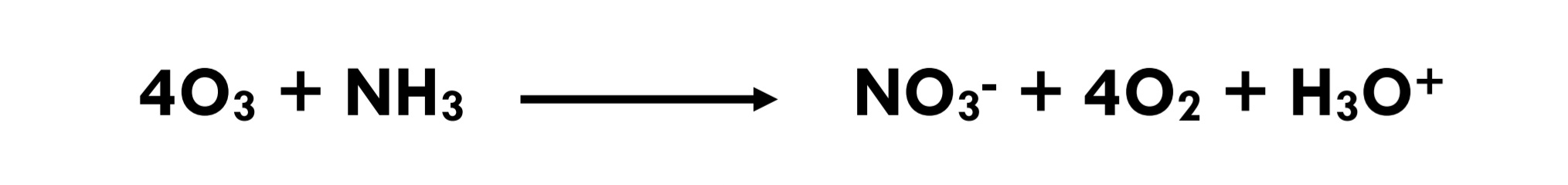
Eq 3.3-15
For disinfection and for oxidation of many organic and inorganic contaminants (Eq 3.3-13, 14, 15) in drinking-water, the kinetics of ozone reactions are favourable; on the other hand, for many difficult-to-oxidize organic compounds, such as chloroform (CHCl3), the kinetics of ozone oxidation are very slow (Hoigne et al., 1985) [14]
CHLORAMINE BY-PRODUCTS :
Monochloramine is a poor primary disinfectant. Additionally, it is a poor oxidant and is not effective for taste and odour control or for oxidation of iron and manganese. However, because of its persistence, it is an attractive secondary disinfectant for the maintenance of a stable distribution system residual. [15]
The use of disinfectants such as ozone or chlorine dioxide combined with chloramines as a secondary disinfectant appears to be attractive for minimizing DBP formation (Singer, 1994b).[16] Monochloramine is the only useful ammonia-chloramine disinfectant. Dichloramine (NHCl2) and nitrogen trichloride (NCl3) are too unstable to be useful and highly malodorous.
TOXICOLOGY OF DISINFECTANT BY-PRODUCTS
We have previously discussed the harmful effects of disinfectants and their reactions to form disinfectant by-products. Various studies have proved the dire biological effects of DPBs.

Tab 4-1: By-products and their Effects
TRIHALOMETHANES :
(Tab 4-1) Trihalomethanes (or THMs) are the most pervasive by-products of disinfection by chlorine. THMs are volatile liquids at room temperature, thus vaporise during water usage. So, inhalation becomes the primary source of exposure route, in addition to ingestion. Major THMs which have been extensively observed are: Chloroform, Bromodichloromethane (BDCM), Dibromochloromethane (DBCM), and Bromoform.
Fatal acute chloroform intoxication via inhalation was reported to be the root cause for cardiomyocyte fragmentation and waviness which indicated acute heart failure, possibly due to arrhythmias or cardiac depression (Harada et al., 1997). [17]
IARC (International Agency for Research on Cancer) concluded that there is sufficient evidence for carcinogenicity of BDCM in experimental animals but inadequate evidence for carcinogenicity of BDCM in humans. Thus, it was assigned to be possibly carcinogenic to humans. (IARC, 1991, 1999) [18] [19]
No strong evidence of acute, short-term, chronic, reproductive, and developmental toxicity; neurotoxicity; toxicity in humans; carcinogenicity and mutagenicity were reported for DBCM.
Bromoform was used in the late 19th and early 20th century as a sedative for children with whooping cough. Patients were given 1 drop 3-6 times a day. (Burton-Fanning, 1901). [20] A few rare instances of death or near-death were reported, cause being accidental overdose. (Dwelle, 1903) [21]
HALOACETICACIDS :
(Tab 4-1) Haloacids like Dichloroacetate (DCA) and Trichloroacetate (TCA) are present at higher concentrations than its counterpart,
Monochloroacetate in drinking water treated with chlorine. Dichloroacetate, widely known as dichloroacetic acid, is a salt and is found ionised in water. DCA was first administered as an oral hypoglycaemic agent to patients suffering from diabetes mellitus (Stacpoole et al., 1978). [22] But soon DCA was not in use due to the study by Moore et al.(1979b) and Stacpoole(1989) as it induced reversive polyneuropathy in patients. [23] [24]
Like DCA, TCA also exists as in salt form in the pH of drinking water.[25]Being a strong acid, it is known that contact of TCA with skin can form acid burns, and the ingestion of the same can damage the GI tract. [26]
HALOALDEHYDES AND HALOKETONES :
The most important compound found in drinking water is trichloroacetaldehyde (or chloral hydrate). Trichloroacetaldehyde is hydrated in water and in the body to form a well-known sedative-hypnotic chloral hydrate.
Chloral hydrate is primarily known for its depressant effects on the central nervous system (Gilman et al., 1991) [27]. The most important acute toxic effect is the production of cardiac arrhythmias. Lower doses of chloral hydrate have been associated with some adverse side-effects. A study of new-borns administered chloral hydrate indicated a high incidence of direct hyperbilirubinaemia (Lambert et al., 1990) [28]. There have been occasional reports of liver damage induced by high doses of chloral hydrate by ingestion (van Heijst et al., 1977; Gilman et al., 1991) [27] [29].
In general, aldehydes are irritant chemicals, and substitution of chlorine generally increases this irritancy. Concentrations of chloroacetaldehyde as low as 0.02% produced intradermal irritation, 7.5% produced rather severe dermal irritation and 0.03% irritated the eyes of rabbits (Lawrence et al., 1972). [30]
There are considerable data on the mutagenic properties of various halogenated aldehydes and ketones. there is a potential carcinogenic hazard associated with the halogenated aldehydes. Only a single compound, chloroacetaldehyde, was evaluated as a carcinogen in a lifetime study, and only one dose level was studied. It appears to be more potent as a carcinogen than the corresponding THM and HAA by-products.
CHLORATE :
There have been sporadic reports of poisoning with sodium or potassium salts of chlorate (Temperman & Maes, 1966; Mengele et al., 1969; Yoshida et al., 1977; Bloxham et al., 1979; Helliwell & Nunn, 1979; Steffen & Seitz, 1981). [31] [32] [33] [34] [35] [36] Most of these cases involved ingestion of preparations of sodium chlorate used for pesticidal purposes. There was generally evidence of oxidative damage to erythrocytes, methaemoglobin formation and the renal complications of haemolytic anaemia.
BROMATE :
(Tab 4.5-1) Human poisonings have been associated with the ingestion of sodium bromate and potassium bromate. Many of these poisonings result from accidental or deliberate ingestion of preparations used as neutralizers in permanent wave kits (Warshaw et al., 1985; Lue et al., 1988).[37] [38] Clinical signs of bromate poisoning include anaemia and haemolysis, renal failure and hearing loss. Loss of hearing appears to be more common in adults than in children (Lichtenberg et al., 1989) [39]. The hearing loss and renal failure can have a prolonged course in some, but not all, people poisoned by bromate (Kuwahara et al., 1984) [40].
REASON FOR CHANGE
By now, we have accumulated an astronomical amount of data and evidence that suggests that the chemical disinfectants we use in day-to-day life is a double-edged sword. Although it is efficient in killing microbes, but we still have to pay the price for prolonged exposure to it. Hence, it is imperative that we take immediate action to lessen its impact.
Even with strict laws regarding the manufacture and distribution of disinfectants, there are many factories where appropriate measures for protection of employees are absent. This can be explained by negligent implementation of laws by the government, lack of awareness between workers and almost no accountability taken by the employers. Even if this issue is solved, a major problem still remains. The manufacturing process and acquisition of raw materials for disinfectants have an undesirable effect on the environment.
To overcome this, we need to adopt a more eco-friendly process which aligns with the Sustainability Development Goals.

Fig 5: Sustainable Development Goals
(Fig 5) The Sustainable Development Goals (SDGs) were born at the United Nations Conference on Sustainable Development in Rio de Janeiro in 2012. The objective was to produce a set of universal goals that meet the urgent environmental, political, and economic challenges facing our world.
Sustainable development has been defined in many ways, but the most frequently quoted definition is from Our Common Future, also known as the Brundtland Report :
"Sustainable development is development that meets the need of the present without compromising the ability of future generations to meet their own need"
With the help of this post, I'd like to assist this process of changing and conserving our environment.
The primary goals that will be focussed upon are :
Goal 3 : Good Health and Well Being [of the workers and users]
Goal 6 : Clean Water and Sanitation
Goal 8 : Decent Work and Economic Growth [for companies trying to be more sustainable]
Goal 11 : Sustainable Cities and Communities [by lessening the negative impact of synthetic disinfectants and DBPs]
Goal 12 : Responsible Consumption and Production [for the betterment of ourselves anf the next generations]
Goal 13 : Climate Action [by decreasing the carbon footprint of the industries]
SUSTAINABLE ALTERNATIVES
Although there does not exist an alternative of disinfectant for all possible uses, there are some that we can use at our households.
Internet is filled with recipes to remove stain and grease, brighten pots and clean tiles. Some of the ingredients commonly used are vinegar, baking soda, lemon, borax, washing soda, rubbing alcohol and hydrogen peroxide. The combinations of these substances have been proven to be effective, and can be readily tested at your home.
One of the most important place for disinfectant use is in the medical field. From surgery to hospital beds, everything needs to be clean to prevent any further infection. Thus an excessive amount is used to ensure that. This has now led to the problem of superbugs, or strains of microorganisms that have evolved to be resistant to the synthetic disinfectants used. As the use of disinfectant is futile, it may lead to a serious problem in the far future. Thus, I believe that rather than continuing on this path, we should shift our attention to plant or other biological based solutions.
This change is a long one and won't be done in a single night. So, we should further scrutinize the laws, fine tune them and make sure they are implemented correctly. Also, we should not irresponsibly use the disinfectants for our and the environment's wellbeing.
One such sustainable alternative to disinfectants is Aloe vera. The second part of my project discusses as to why Aloe can be used and how does it disinfect. You can read more about it on the post 'Aloe Vera : A Sustainable Disinfectant'.
Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants Hazards of Disinfectants
Hazards of Disinfectants
Hazards of Disinfectants
REFERENCES
[1] NCI Dictionary of Cancer Terms. National Cancer Institute. Retrieved from https://www.cancer.gov
[2] Amy, G., Bull, R., Craun, G.F., Pegram, R.A., & Siddiqui, M. (2000). Environmental Health Criteria for Disinfectants and Disinfectant by-product. United Nations Environment Program, International Labour Organization, & World Health Organization. EHC 216, Chapter 1: Summary and Evaluation. Retrieved from http://www.inchem.org/
[3] Amy, G., Bull, R., Craun, G.F., Pegram, R.A., & Siddiqui, M. (2000). Environmental Health Criteria for Disinfectants and Disinfectant by-product. United Nations Environment Program, International Labour Organization, & World Health Organization. EHC 216, Chapter 1: Summary and Evaluation, Section 1.3 Toxicology of disinfectants and disinfectant by-product. Retrieved from http://www.inchem.org/
[4] Amy, G., Bull, R., Craun, G.F., Pegram, R.A., & Siddiqui, M. (2000). Environmental Health Criteria for Disinfectants and Disinfectant by-product. United Nations Environment Program, International Labour Organization, & World Health Organization. EHC 216, Chapter 1: Summary and Evaluation, Section 1.2 Kinetics and metabolism in laboratory animals and humans, Sub-section 1.2.1 Disinfectants. Retrieved from http://www.inchem.org/
[5] Press Trust of India (18 April 2020). Spraying of disinfectant on people 'physically and psychologically harmful': Health ministry. The Economic Times. Retrieved from https://economictimes.indiatimes.com/
[6] Yarington CT (1970) The experimental causticity of sodium hypochlorite in the esophagus. Ann Otol Rhinol Laryngol, 79(5): 895-988
[7] Das R & Blanc PD (1993) Chlorine gas exposure and the lung: A review. Toxicol Ind Health, 9: 439-455.
[8] Eaton JW, Kolpin CF, & Swofford HS (1973) Chlorinated urban water: a cause of dialysis induced hemolytic anemia. Science, 181: 463-464
[9] Amy, G., Bull, R., Craun, G.F., Pegram, R.A., & Siddiqui, M. (2000). Environmental Health Criteria for Disinfectants and Disinfectant by-product. United Nations Environment Program, International Labour Organization, & World Health Organization. EHC 216, Chapter 1: Summary and Evaluation, Section 1.1: Chemistry of disinfectants and disinfectant by-product. Retrieved from http://www.inchem.org/
[10] Amy, G., Bull, R., Craun, G.F., Pegram, R.A., & Siddiqui, M. (2000). Environmental Health Criteria for Disinfectants and Disinfectant by-product. United Nations Environment Program, International Labour Organization, & World Health Organization. EHC 216, Chapter 2: Chemistry of Disinfectant and Disinfectant By-Products, Section 2.1: Physical and Chemical Properties of Common disinfectants, Sub-Section 2.2.1: Chlorine. Retrieved from http://www.inchem.org/
[11] Amy, G., Bull, R., Craun, G.F., Pegram, R.A., & Siddiqui, M. (2000). Environmental Health Criteria for Disinfectants and Disinfectant by-product. United Nations Environment Program, International Labour Organization, & World Health Organization. EHC 216, Chapter 2: Chemistry of Disinfectant and Disinfectant By-Products, Section 2.4: Mechanisms involved in the formation of disinfectants by-products, Sub-Section 2.4.2: Chlorine Dioxide. Retrieved from http://www.inchem.org/
[12] Amy, G., Bull, R., Craun, G.F., Pegram, R.A., & Siddiqui, M. (2000). Environmental Health Criteria for Disinfectants and Disinfectant by-product. United Nations Environment Program, International Labour Organization, & World Health Organization. EHC 216, Chapter 2: Chemistry of Disinfectant and Disinfectant By-Products, Section 2.1: Physical and Chemical Properties of Common disinfectants, Sub-Section 2.2.2: Chlorine Dioxide. Retrieved from http://www.inchem.org/
[13] Tratnyek, P. G., & Hoigné, J. (1994). Kinetics of reactions of chlorine dioxide (OCIO) in water-II. Quantitative structure-activity relationships for phenolic compounds. Water Research, 28(1), 57-66. https://doi.org/10.1016/0043-1354(94)90119-8
[14] Hoigne J, Bader H, Haag WR, & Staehelin J (1985) Rate constants of reactions of ozone with organic and inorganic compounds in water: III. Inorganic compounds and radicals. Water Res, 19(8): 993-1004
[15] Amy, G., Bull, R., Craun, G.F., Pegram, R.A., & Siddiqui, M. (2000). Environmental Health Criteria for Disinfectants and Disinfectant by-product. United Nations Environment Program, International Labour Organization, & World Health Organization. EHC 216, Chapter 2: Chemistry of Disinfectant and Disinfectant By-Products, Section 2.2: Physical and Chemical Properties of Common disinfectants, Sub-Section 2.2.4: Chloramine. Retrieved from http://www.inchem.org/
[16] Singer PC (1994b) Impacts of ozonation on the formation of chlorination and chloramination by-products. Denver, Colorado, American Water Works Association Research Foundation.
[17] Harada K, Ichiyama T, Ikeda H, Ishihara T, & Yoshida K (1997) An autopsy case of acute chloroform intoxication after intermittent inhalation for years. Nippon Hoigaku Zasshi, 51(4): 319-323
[18] IARC (1991) Chlorinated drinking-water; chlorination by-products; some other halogenated compounds; cobalt and cobalt compounds. Lyon, International Agency for Research on Cancer (IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans, Volume 52)
[19] IARC (1999) Re-evaluation of some organic chemicals, hydrazine, and hydrogen peroxide. Lyon, International Agency for Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 71)
[20] Burton-Fanning FW (1901) Poisoning by bromoform. Br Med J, 18 May: 1202-1203
[21] Dwelle EH (1903) Fatal bromoform poisoning. J Am Med Assoc, 41: 1540
[22] Stacpoole PW, Moore GW, & Kornhauser DM (1978) Metabolic effects of dichloroacetate in patients with diabetes mellitus and hyperlipoproteinemia. N Engl J Med, 298: 526-530
[23] Moore GW, Swift LL, Rabinowitz DR, Crofford OB, Oates JA, & Stacpoole PW (1979b) Reduction of serum cholesterol in two patients with homozygous familial hypercholesterolemia by dichloroacetate. Atherosclerosis, 33: 285-293
[24] Stacpoole PW (1989) The pharmacology of dichloroacetate. Metabolism, 38: 1124-1144
[25] IARC (1995) Dry cleaning, some chlorinated solvents, and other industrial chemicals. Lyon, International Agency for Research on Cancer (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 63)
[26] Amy, G., Bull, R., Craun, G.F., Pegram, R.A., & Siddiqui, M. (2000). Environmental Health Criteria for Disinfectants and Disinfectant by-product. United Nations Environment Program, International Labour Organization, & World Health Organization. EHC 216, Chapter 4: Toxicology of Disinfectant By-Product, Section 4.2: Haloacids, Sub-Section 4.2.2: Trichloroacetic acid (Trichloroacetate), Sub-Sub-Section: Toxicity in Humans. Retrieved from http://www.inchem.org/
[27] Gilman AG, Rall TW, Neis AS, & Taylor P (1991) Goodman and Gilman’s pharmacological basis of therapeutics, 8th ed. New York, Pergamon Press, pp 364-365
[28] Lambert GH, Muraskas J, Anderson CL, & Myers TF (1990) Direct hyperbilirubinemia associated with chloral hydrate administration in the newborn. Pediatrics, 86: 277-281
[29] van Heijst AN, Zimmerman AN, & Pikaar SA (1977) [Chloral hydrate - the forgotten poison.] Ned Tijdschr Geneesk, 121(40): 1537-1539 (in Dutch)
[30] Lawrence WH, Dillingham EO, Turner JE, & Autian J (1972) Toxicity profile of chloroacetaldehyde. J Pharm Sci, 61: 19-25
[31] Temperman J & Maes R (1966) Suicidal poisoning by sodium chlorate: A report of three cases. J Forensic Med, 13: 123-129
[32] Mengele K, Schwarzmeier J, Schmidt P, & Moser K (1969) [Clinicalfeatures and investigations of the erythrocyte metabolism in case ofintoxication with sodium chlorate.] Int J Clin Pharmacol, 2: 120-125 (in German)
[33] Yoshida Y, Hirose Y, Konda S, Kitada H, & Shinoda A (1977) A cytological study of Heinz body-hemolytic anemia. Report of a case of sodium chlorate poisoning complicated by methemoglobinemia and acute renal failure. Acta Haematol Jpn, 40: 147-151
[34] Bloxham CA, Wright N, & Hoult JG (1979) Self-poisoning by sodium chlorate: Some unusual features. Clin Toxicol, 15: 185-188
[35] Helliwell M & Nunn J (1979) Mortality in sodium chlorate poisoning. Br Med J, April 28(6171): 1119
[36] Steffen C & Seitz R (1981) Severe chlorate poisoning: Report of a case. Arch Toxicol, 48: 281-288
[37] Warshaw BL, Carter MC, Hymes LC, Bruner BS, & Rauber AP (1985) Bromate poisoning from hair permanent preparations. Pediatrics, 76: 975-978.
[38] Lue JN, Johnson CE, & Edwards DL (1988) Drug experience: Bromate poisoning from ingestion of professional hair-care neutralizer. Clin Pharm, 7: 66-70
[39] Lichtenberg R, Zeller WP, Gatson R, & Hurley RM (1989) Clinical and laboratory observations: Bromate poisoning. J. Pediatr, 114: 891-894
[40] Kuwahara T, Ikehara Y, Kanatsu K, Doi T, Nagai H, Nakayashiki H, Tamura T, & Kawai C (1984) Two cases of potassium bromate poisoning requiring long-term hemodialysis therapy for irreversible tubular damage. Nephron, 37: 278-280
Join Weekly Health Newsletter
Every week, I will share with you information about Natural Health, Diet, Exercise, Yoga & Wellness.

0 comments