On 15th October, 2025, the Food Safety and Standards Authority of India (FSSAI), has issued a directive requiring all food and beverage companies to cease using the term “ORS” (Oral Rehydration Solution) on their products. The order extends to all instances where the term appears on its own, with any prefix or suffix, or as part of a trademark or brand name.
On this note, I would like to expand more on the topic of Oral Rehydration Solution, its constituents and uses as directed by WHO while touching on the clarification issued by FSSAI.
Join Weekly Health Newsletter
Every week, I will share with you information about Natural Health, Diet, Exercise, Yoga & Wellness.
BASIC TERMINOLOGIES
Dehydration
Loss of water and dissolved salts from the body, occurring, for instance, as a result of diarrhoea.
Rehydration
The correction of dehydration.
Oral Rehydration Therapy (ORT)
The administration of fluid by mouth to prevent or correct the dehydration that is a consequence of diarrhoea.

ORAL REHYDRATION SALT SOLUTION

Oral Rehydration Salts (ORS) is the internationally recognized non-proprietary name for a scientifically formulated glucose-electrolyte solution developed in 1969. It was approved, recommended, and distributed by the World Health Organization (WHO) and UNICEF as a life-saving medical formulation for the prevention and treatment of dehydration caused by diarrhea and other conditions leading to fluid loss. It remains a cornerstone of primary healthcare and is classified as an essential medicine by the WHO for its proven safety, affordability, and effectiveness.
Constituents
Given below is the list of ingredients present in the New ORS solution as advised by WHO.
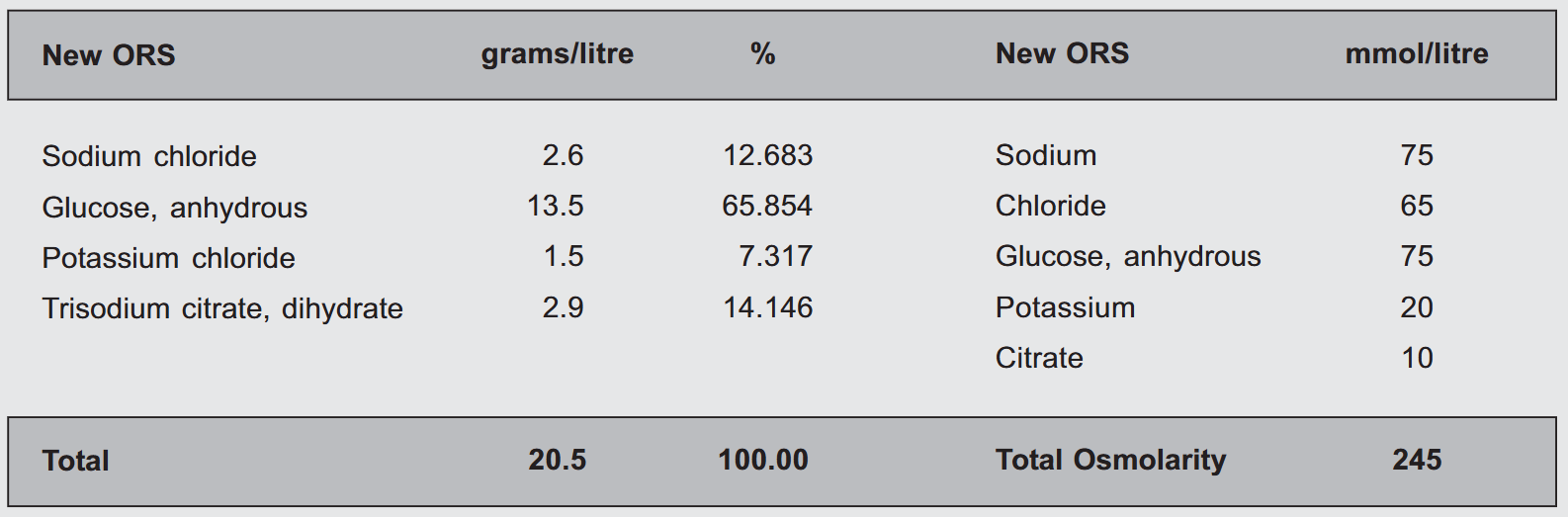
Dissolution of an ORS sachet in drinking water yields the following concentrations :

Difference between the Old and the New
The 'Old' ORS formulation is known as the Standard WHO ORS whereas the 'New' one is called the Reduced Osmolarity ORS. Previously, the ORS had a total osmolarity of 311 mOsm/l, but a consultative technical meeting held in New York (USA) in July 2001 reviewed various studies evaluating this approach. During the meeting, experts provided technical recommendations to WHO and UNICEF regarding the efficacy and safety of reduced osmolarity ORS for treating children with acute non-cholera diarrhea, as well as adults and children suffering from cholera.
These studies showed that the efficacy of ORS solution for treatment of children with acute non-cholera diarrhoea is improved by reducing its osmolarity. In a combined analysis of this study and studies with other reduced osmolarity ORS solutions stool output was also reduced by about 20% and the incidence of vomiting by about 30%.
However, it remains uncertain whether this finding can be confidently applied to patients with severe cholera-induced diarrhea, in whom electrolyte losses are extensive. In such cases, the risk of developing hyponatremia may be heightened, potentially leading to adverse clinical outcomes and diminishing the benefits of using reduced osmolarity ORS.
Role of the Constituents
- Glucose : Facilitates the absorption of sodium (and hence water) on a 1:1 molar basis in the
small intestine - Sodium and Potassium : To replace the body losses of these essential ions during
diarrhoea (and vomiting) - Citrate : Corrects the acidosis that occurs as a result of diarrhoea and dehydration.
Uses
DEHYDRATION :
While the mortality rate among children under five suffering from acute diarrhea has declined remarkably—from about 4.5 million deaths annually in 1979 to 1.6 million in 2002—acute diarrhea still remains a significant cause of illness and death among children in developing countries.
The introduction of Oral Rehydration Salts (ORS) and Oral Rehydration Therapy (ORT) by UNICEF and WHO in the late 1970s represented a major milestone in global child health. During the 1990s, these therapies were estimated to have saved more than one million young lives each year by effectively preventing deaths from dehydration caused by diarrhea.
Nevertheless, recent trends indicate a concerning decline in both awareness and the correct use of home-based treatments for diarrhea, including ORT, in several countries—threatening to undermine decades of progress in reducing child mortality.
Hence, the healthcare workers are advised to : [Detailed]
Treat dehydration with ORS solution (or with an IV electrolyte solution in cases of severe dehydration) &
Provide children with 20 mg per day of zinc supplementation for 10–14 days (10 mg per day for infants under six months old)
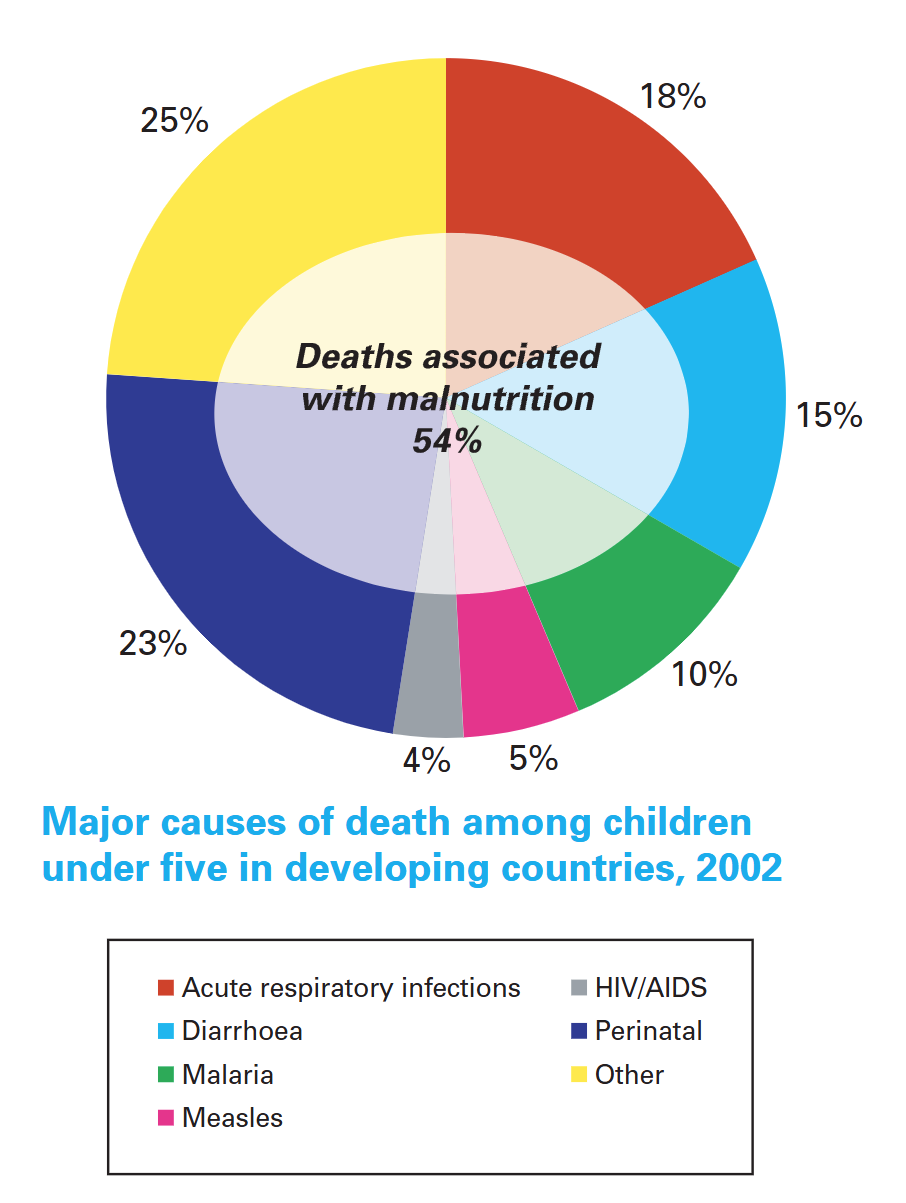
For cause-specific mortality: The World Health Report2003, WHO, Geneva. For malnutrition: Pelletier, D. L., E. A. Frongillo, and J. P. Habicht, ‘Epidemiologic evidence for a potentiating effect of malnutrition on child mortality’,American Journal of Public Health, vol. 83, no. 8, August 1993, pp. 1130-1133.
OTHERS :
In a study about ORS as alternative to IV fluids in adult burn patients with >15% TBSA burn injuries, it was observed that :
- there were no major significant differences between the study group and control group.
- Even where there was a significant difference, apart from blood pressure in the first hour of the first day, the study group never crossed safe limits for other parameters.
- WHO-ORS with 5gm salt tablets, given according to Sørenson’s formula, is a safe and efficient alternative for IV resuscitation.
- It could even be a substitute, particularly in low resource settings and fire disasters.
FSSAI

FSSAI, or the Food Safety and Standards Authority of India, is an independent statutory body responsible for establishing and enforcing food standards in India, ensuring that all food products are safe, hygienic, and fit for human consumption.
Previous Stance on ORS
Earlier directives from the Centre, issued on July 14, 2022, and February 2, 2024, permitted the use of the term “ORS” on product labels as part of a trademark with a prefix or suffix. This was allowed only provided the label included the disclaimer: “The product is NOT an ORS formula as recommended by WHO.”
Clarification dated 15th Oct, 2025
FSSAI has reiterated that, following a fresh review, the use of the term “ORS” in any form—whether as part of a trademark or otherwise—on products like fruit-based, non-carbonated, or ready-to-drink beverages violates the Food Safety and Standards Act.
The authority emphasized that such labeling can mislead consumers by being false, deceptive, ambiguous, or inaccurate.
Additionally, FSSAI warned that products in breach of this rule may incur penalties under Sections 52 and 53 of the Food Safety and Standards Act, 2006.
Dr. Sivaranjani Santosh
Dr Sivaranjani Santosh, paediatrician, says the following...
“It's a huge relief. That means now I know that no child will die, no adult will die because of worsening of diarrhoea, because of these drinks. ORS is supposed to save lives. It's a wonder drug of the 20th century. It's supposed to save lives. It's supposed to rehydrate us. It is like Amrut for us. And here, people have labelled their high sugar drinks as ORS with a suffix or prefix. And for the past 14 years, they have been cheating the public, deceptive labelling, unethical marketing, pharmacies, hospitals, schools, everywhere,”
In 2022, Dr. Santosh filed a PIL in the Telangana High Court against companies labeling sweetened fruit juices as ORS without meeting WHO standards, citing high sugar content and inconsistent electrolytes.
The case drew the attention of then Health Secretary Rajesh Bhushan, prompting judicial and regulatory review. FSSAI initially restricted the use of ‘ORS’ on labels and ads on April 8, 2022, but temporarily relaxed the rule on July 14, 2022, allowing companies with registered trademarks to continue using the term until a final ruling by the Controller General of Patents, Designs and Trademarks.


Dr. Santosh’s eight-year campaign shed light on companies marketing sugar-heavy drinks as ORS, falsely suggesting health benefits. Her advocacy emphasized the risks these products pose, especially to children and people with diabetes. And finally, FSSAI has now given is new directive in favour of her campaign.

0 comments